Urinary M
1/320
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
321 Terms
True or false
Lab work on dogs shows he is azotemic. this confirms he has glomerular disease
False
protienuria with inactive sediment is the hallmark of glomerular disease
What does an inactive sediment mean
a urine sample that is <5 white blood cells / high powr filed (hpf) and <10 red blood cells /hpf were observed
How can you specifically diagnose what type of glomerular disease a animal has (the only way)
Only biopsy which is not commonly done though expensive and have to send it off
Most common cause of proteinuria
Lower urinary tract disease (bacterial cystitis)
No need to run UPC ratio if active sediment present (because it will go away once infection clears)
Four cirteria that define nephrotic syndrome and must have all four
proteinuria
hypoalbuminamia
hypercholesterolamia
ascites/edema
How does leaking protein cause damage?
Protein escapes into the mesangium triggering inflammation
body response and you get formation of scar tissue
this pretty much kills the glomerulas when you have excessive scar tissue
the reamining gloemrulus have to pick up the load which also increases the pressure progressing the disease (snowball effect)
What is the most common undelrying cause of glomerulonephritis?
Immunologic injury
this could be due to circulating antigen antibody complex depositing into the kidney or in situ
Note: many diseases can cause proteinuria so rule out any underlyig causes such as cancer or infectious diseases (most cases are idopathic)
Glomerular disease is more common in cats or dogs?
Dogs
Familial glomerular disease of soft coated wheaton terriers, Bernese moutain dogs, brittany spaniels
Membranoproliferative GN
Familial glomerular disease of English cocker spaniels, Samoyed, Doberman pinchers and bull terriers
Basement membrane disorders
Amyloidosis is a diverse group of diseases characterized by extracellular deposition of protein, subunits that form Beta pleated sheets
Amyloidosis can be classified by what two categories
Distribution of deposits
systemic (most common**)
Localized (pancreatic islet cells in cats)
nature of responsible protein
reactive (AA) most common
Most common nature of amyloidosis is reactive systemic amyloidosis
can be associated with chronic infectious and non-infectious inflammatory disease and neoplasia
What is the most common cases in dogs and cats?
Idiopathic or familial
Three dogs most commonly get familiar reactive systemic amyloidosis
Sharpie
Beagle
English foxhound
Three common cats that get familiar reactive systeic amyloidosis
Abyssinian
Siamese
Oriental short hair
Tissue distribution of amyloid deposits in dogs and cats widespread HOWEVER clinical signs are due to what organ involvement
Kidney involvement, causing chornic kidney disease
Amyloids like to deposit in the kidney
What is the exception to amyloid deposits concerning tissue tropism of amyloid proteins
In sharpie dog, Siamese and oriental shorthair cats, you can also get severe liver involvement causing liver rupture and hemoabdomen
With reactive systemic amyloidosis they commonly have tropism for kidneys
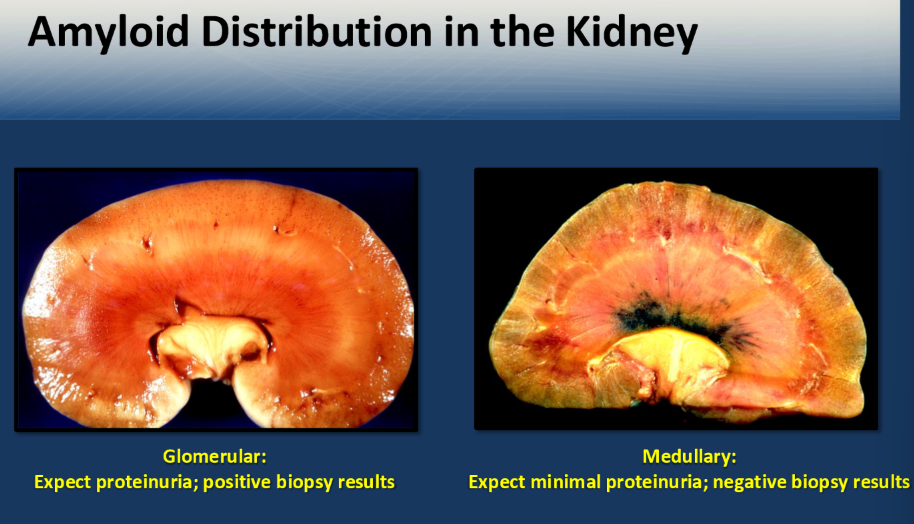
Which animal does the distribution of deposits within the kidney involve the glomerulus more so than medullary (exam)
Dogs
Note: including human and horses
important = you will get deposition in both, however with dogs you will see them more so in the gloemrular (important concept to understand)
What exception of dogs has more medullary involvement compaired to glomerular
Sharpei dog
With reactive systemic amyloidosis which animal has more medullary involvement rather than glomerular
Cats (including Abyssinian) and Sharpei
note: cow
Dogs with amyloid deposition present with increased UPC because gloemrular is damaged
How do cats present if they get mainly medullary amyloidosis
Chronic kidney disease (tubules are affected) - present with isosthenuria
which will give you a negative biopsy result for amyloid distribution
Glomerular or medullary
Medullary
you cant go in that deep to get biopsy
Mean age for Shar-Pei dog that gets amyloidosis
Four years old (young dog)
more commonly black and fawn breed
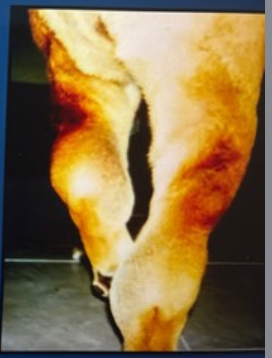
Shar-Pei dog presents with isosthenuria and self limiting fever with tibial joint swelling
What should be on your Ddx?
Amyloidosis with kidney involvement
this is known as Shar-Pei fever
they will have signs consistent with CKD and have history of recurrent acute self-limiting fever adn tibial joint swelling
Remember, they may also have severe liver involvement causing icterus, or Hemo abdomen due to liver rupture
How much loss of function of teh kidney before an animal can become azotemic (excludign dehydration)
¾
so if the animal is isothenuric with azotemia this means that they have lost a significant function of the nephron
true or false
Glomerular disease is often a disease of middle aged to older animals
True
There is no gender predilection for glomerular disease, however which gender of cats usually has GN
Males
Six possible presentations of animals where you can also find glomerular disease
CKD (most common)
underlying infections, inflammatory or neoplastic disorder (glomerular disease due to antigen antibody complex)
proteinuria as a incidential finding
nephrotic syndrome
thromboembolism (loss of antithrombin 3, liver making more clotting factors)
sudden blindness (hypertension, causing retinal detachment)
What is the best way to determine significance of proteinuria
Running a UPC
Three differentials for hypoalbuminaemia
protein loosing neuropathy
liver disease
Protein loosing endocrine (cushings, hypothyroid)
Normal UPC
<0.3-0.4
on average, the highest UPC ratios are seen in patient with what type of liver disorder
Amyloidosis
specifically glomerular amyloidosis
Note: GN can range from normal to over 30 it is variable
Where do you see the lowest UPC ratios
Interstitial renal disease
Magnitude of urinary protein/urinary creatine correlates with severity of glomerular disease in only what type of patient
Non-azotemic patient
once they are azotemic, there is no correlation
When is UPC unreliable? exam
In the presence of pyuria or severe hematuria
If you have a azotemic patient and you see a decrease in their UPC does this mean they are improving?
No
cannot assess because there are azotemic
Conventional dipstick tests can detect protein that is between 10-30 MG/DL
What test can you detect protein levels greater than one but below 10-30 mg/dl
Microabluminuria test
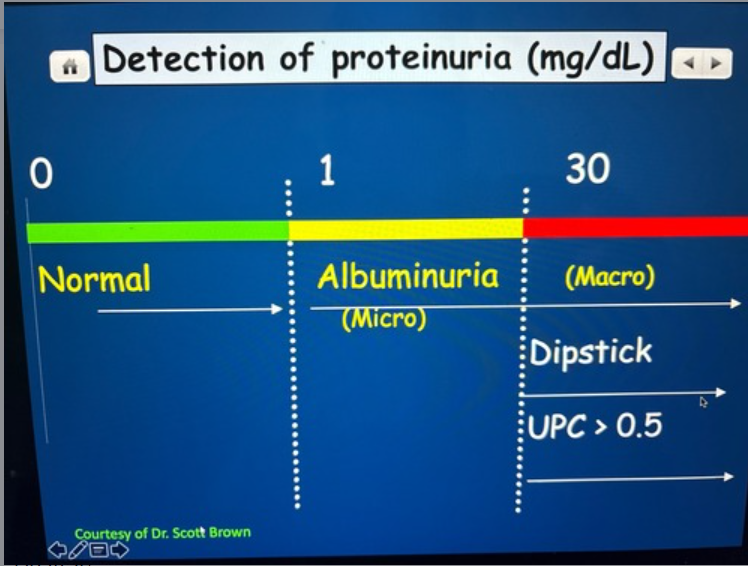
True or false
Microalbuminuria can be an early indicator of vascular endothelial damage however, prognostic values in dogs and cats are uncertain.
True
Two commonly used drugs to decrease protienuria
Angiotensin converting enzyme inhibitor (ACE) (eg. Enalapril, Benazepril)
Angiotensin recptor blocker (Telmisartan)
The goal is to reduce hypertension
ACE inhibitors and ARB inhibitors preferentially act where in the kidney (exam)
Efferent arteriole (this will promote vasodilation)
Decrease glomerular capillary hydrostatic pressure (and proteinuria) by decreasing post-glomerular arteriolar resistance (efferent arteriole)
Note: have the potential to make patient azotemic
During rechecks, you measure, creatinine, potassium, blood pressure and UPC
What do you want to change in the diet with an animal that has glomerular disease?
Lower protein intake
If the animal has a very high amount of hypertenison, what can you also use in addition to your ACE inhibitors and angiotensin receptor blockers?
Amlodipine
Why are patients with glomerular disease high risk for thromboembolism?
They lose antithrombin in urine
this makes them hypercoagulable
Most common drug used to day to prevent thromboembolism
Clopidogrel (platelet inhibitor)
if you rule out infectious, neoplastic diseases, and a biopsy supports glomerulonephritis how do you empirically treat?
Immunosuppressive inflammatory therapy
If owner does not want to do biopsy you can still treat with immunosuppressive therapy
What is the drug of choice for rapidly progressive glomerular disease of an apparent immune-mediated pathogenesis?
Mycophenolate
What is the specific treatment for amyloidosis?
No specific therapy shown to be beneficial
True or false
Hypertension occurs in 50 to 80% of dogs with glomerular disease.
True
True or false
Control of blood pressure can slow progression of renal disease
True
Blood pressure should be measured in all dogs and cats with glomerular disease*** before you do anything else.
Prognosis for amyloidosis and glomerular nephritis
Amyloidosis = poor
Glomerular nephritis = variable (spontaneous remission, stable for months to years, progresses to CKD)
How can acute renal failure be defined?
Abrupt decline in renal function with a recent onset of azotemia
unable to regulate volume and composition of body fluids
Is acute renal failure reversible
Potentially is
Three categories of acute renal failure
Acute pre-renal
Acute intrinsic renal (primary)
acute post renal
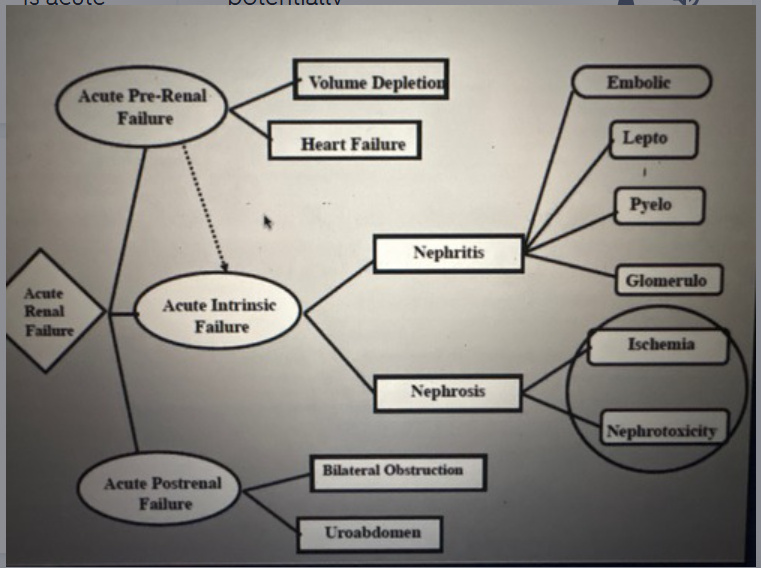
Three common causes of acute intrinsic renal failure
Nephrotoxic nephrosis
Nephritis
Ischemic nephrosis
True or false
Systemic arterial hypertension is not required to cause ischemic nephrosis
True
Lepto affects what two organs
kidney and liver
What is a universal feature of all leptospirosis pathogenic serovars
They have the ability to colonize proximal renal tubules
(results in prolonged renal carrier state, and urine shedding)
If a animla recovers from leptospirosis does it still shed?
Yes, they can shed it in their urine for months to years after infection
Note: wild animals are the reservoirs
How are animals usually exposed to lepto
Through Mucocutaneous contact
Can also penetrate mucosa or abraded skin
Leptospiremia occurs 4 to 12 days post-infection
it can affect the kidney liver or both
Why may you see thrombocytopenia with leptospiremia
causes vasculitis and DIC from widespread acute endothelial injury
Most common cause of acute renal failure/nephritis
Leptospirosis
Exposure to Neuro toxins/ischemia causes ___ injury
Tubular
Ranges from degeneration (nephrosis) to acute tubular necrosis (ATN)
three pathophysiologies of acute intrinsic renal failure (nephrosis)
Afferent arteriolar constriction (vasomotor nephropathy hypertension)
Obstruction (intraluminal or extraluminal)
Tubular backleak (due to inflammation)
How can NSAIDs cause ischemic AIRF
They block Reno prostaglandin synthesis, which is responsible for renal vasodilation
so if you have an animal that is dehydrated or hypovolemic this can cause ischemia (give it only to the healthy dog)
Not directly toxic
Mechanism of injury with nephrotoxic AIRF
Cause direct cell injury rather than ischemia
bind tubular cell membranes, and decrease energy production causing cell death
some also cause renal vasoconstriction
Describe the latent (induction) phase of AIRF
Often not detected
clinical signs are minimal or absent
if the agent is removed normal kidney function returns
How can you define the maintenance phase of AIRF
Suddenly increased serum creatinine that persists despite correction of all pre-renal factors (hydrated)
note: do not know how long this phase can persist
characterized by severe drop in RBF and GFR
even if RBF returns to normal, GFR remains low
true or false
when an animal is in the maintenance phase they become oliguric
False
they can have normal urine output, be oliguric, or polyuric
note: the older definition of AIRF required oliguria for diagnosis but not the new one)
If you remove the insulitng agent that caused the AIRF during the maintenance phase will the kidney return to normal funciton
No
patient experiences a 1-3 week course before restoration of renal function can occur
If a patient is oliguic in the maintenance phase why is it important to still put on a catheter?
Because they can go from oliguric to polyuric during the maintenance phase (better)
During the recovery phase, what two outcomes can occur
You can have complete recovery
partial improvement
return of normal BUN and creatinine = POSSIBLE
partial improvement = CKD
There is no single test to definitively diagnose AIRF
so how do you diagnose it?
History (should be recent and possibly an exposure to something nephrotoxic or ischemic)
physical exam (should have a normal BCS - not a chronic condition)
renal size (should be normal or big not small)
important = get your lab samples before you start treatment (such as fluids)
History:
absence of longstanding PU/PD, potential for renal ischemia or nephrotoxin exposure, oliguria, polyuria, non-specific signs
CBC:
especially PCV at outset
on physical exam with acute renal failure what symptoms would you see?
Uremic symptoms (oral ulcers, uremic breath)
May exhibit back pain (renal pain)
Dehydration
Bradycardia if markedly hyperkalemic
normal to large kidneys
no evidence of lower urinary tract obstruction
Note: absence of pallor to mucous membranes
Do you see anemia with acute renal failure?
No, not early on
this is more common with CKD
How would your total proteins look with acute renal failure?
Normal to elevated (relative to dehydration)
If animal with acute renal failure, also has thrombocytopenia, what should be on top of Ddx:
Lepto
What do you see with urine concentration and glucose in AIRF pateint
Dilute urine (tubules arent working)
USG often 1.007-1.017
Does NOT differentiate AIRF from CKD (both have lwo USG)
Glucosuria (lack of function of glucose transporters in tubules)
What might you see on urine sediment with AIRF
Casts
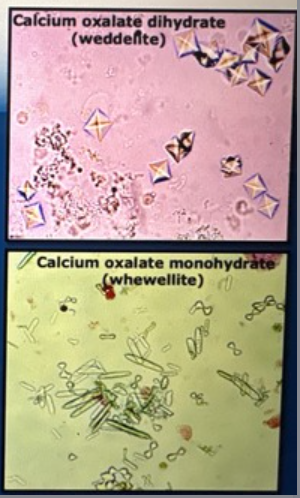
Oxalate crystals if Etheline glycol poisoning
Increased WBC, RBC tubular epithelial cells (reaction to tubular injury)
Note: if bacteria also present suspect pyelonephritis (ascending infection)
may be very active (cylindruria) but absence of casts does not exclude AIRF
increased numbers of oxalate crystals supports ad diagnosis of ethylene glycol poisoning in an oliguric AIRF patient
What do you see on serum biochemistry in AIRF
BUN creatinine phosphorous increase severe (azotemia)
Note: cannot use the magnitude of azotemia to determine if it is AIRF versus CKD ***
OR differentiate between pre-renal, renal, and post-renal
When would you see increase in serum potassium with AIRF
Severely impaired renal excretory function and olguria (decreased urine production will cause the increase in potassium causing the bradycardia)
Note: greater than 5.5
Metabolic acidosis more severe in acute renal failure or chronic kidney disease
Acute renal failure
Note: specifically during maintenance phase
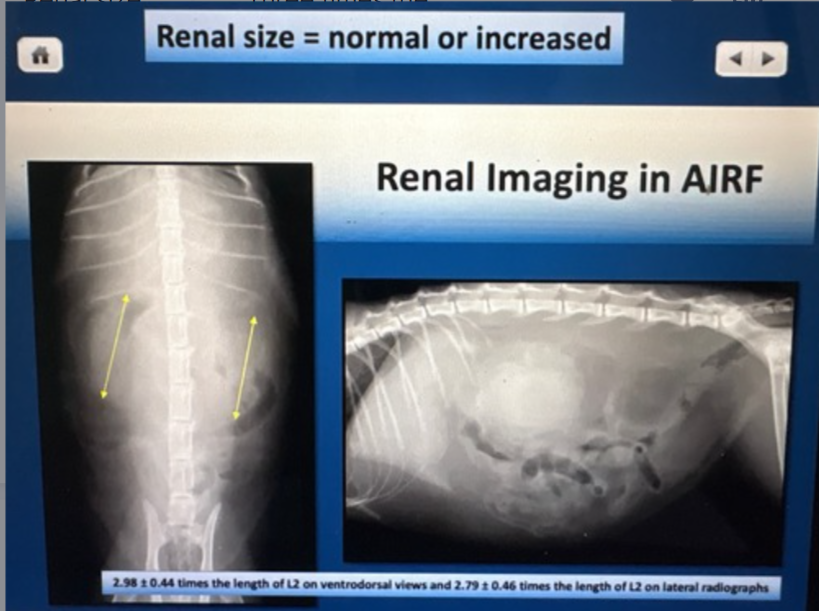
Renal size with AIRF
Three times the length of L2 on ventral dorsal view
Note: normal or increased
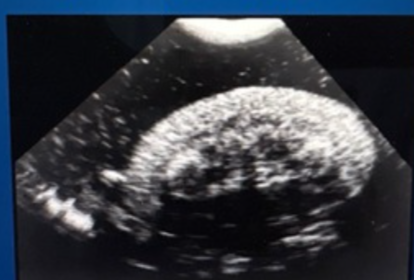
Additional finding on ultrasound with AIRF to support diagnosis
Increased echogenicity of the cortex (loss of corticomedullary distinction)
Note: normal ultrasound does not exclude AIRF
What is the only cause of acute renal failure that can be inferred from ultrasound
Ethylene glycol intoxication
severe hyperechogenicity on ultrasound suggests possibility of ethylene glycol intoxication
Which animals will have an enlarged parathyroid gland CKD or AIRF
CKD
secondary renal hyper renal parathyroidism
Possible cause if nephritis versus glomerulonephritis
nephritis = Leptospirosis
glomerular nephritis = Lyme disease (Borrelia)
Leptospirosis prognosis
Good with adequate supportive fluid and penicillin started early
True or false
AIRF patient with SEVERE baseline azotemia during the maintenance phase often are successfully managed without dailysis
True (dialysis increases prognosis
over 80% of patience with AIRF and high level azotemia either die or be euthanized
Main reasons animals with AIRF die or are euthanized
Hyperkalemia (animal is oliguric - potassium level increases substantively)
metabolic acidosis (owner elects euthanasia)
severe azotemia
overhydration and pulmonary edema due to vigorous fluid therapy
How to measure volume of IV fluid fluids when treating AIRF
Separate 24 hours into four-hour blocks
account for insensible losses 20 mg/kg
add the measured urine output for the proceeding for hours
(20 ml/kg/6 + measured urine output preceding four hours
No “quick fix” (may require several weeks)
How can you treat the hyperkalaemic aspect?
Insulin plus glucose (must include glucose)
Calcium gluconate
What do you need to do if hyperkalemia persist even after attempting to treat it
Must proceed to dialysis
What medication decreases shedding of leptospirosis and what can you give to treat carrier state?
Decreased shedding = penicillin
eliminate carrier state = doxycycline
What enzyme converts Etheline glycol to glycolate
Alcohol dehydrogenase ( so this is where you intervene)
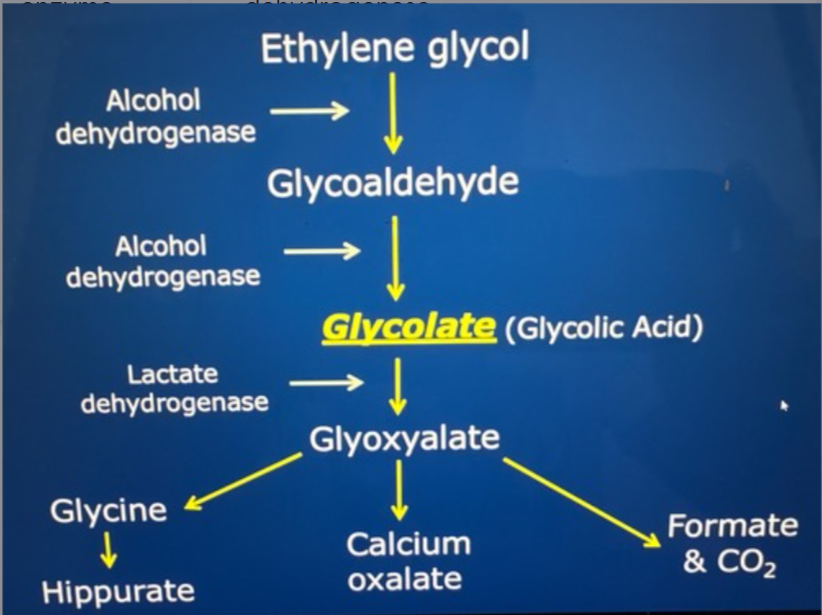
Stages of Etheline glycol poisoning (exam)
Neurologic (30 minutes - 12 hours)
ataxia, stupor, seizures, coma
cardio pulmonary (12 hours - 24 hours)
Tachycardia, tachypnea
renal (24 hours - 72 hours)
initial PD/PU
progresses to profound oligo-anuric AIRF (almost always fatal once established
What metabolite of ethylene glycol poisoning causes tubular damage
Calcium oxalate crystals (tubular back leak, obstruction, edema compromising RBF)
Why can you get false negatives and false positives with ethylene glycol measurement tests
False negatives = test done too early or too late
false positives = other items such as propylene glycol, glycerol and metaldehyde can trigger it to be positive
Clinical signs that are resumptive diagnosis for eg. poisoning
Alcohol like intoxication
PU/PD to oliguria
renal pain
progression to coma
What findings will you see that can help you diagnose eg. poisoning on lab work
Dilute urine
oxalate crystalluria
anion gap metabolic
acidosis
progressive azotemia
hypocalcemia
For best outcome of treating eg poisoning treat within how many hours of ingestion
Four hours
Do not wait for definitive test results start and induce vomiting and gastric lavage with activated charcoal
Attempt to stimulate urine production with furosemide
What is the definitive treatment for eg poisoning to prevent metabolism of ethylene glycol
Fomepizole (4-methylpyrazole)
compeititve inhibitor