Specific heat capacity: Energy: Physics: GCSE (9:1)
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
ΔE = mcΔθ
The equation linking the change in thermal energy, mass, specific heat capacity and temperature change
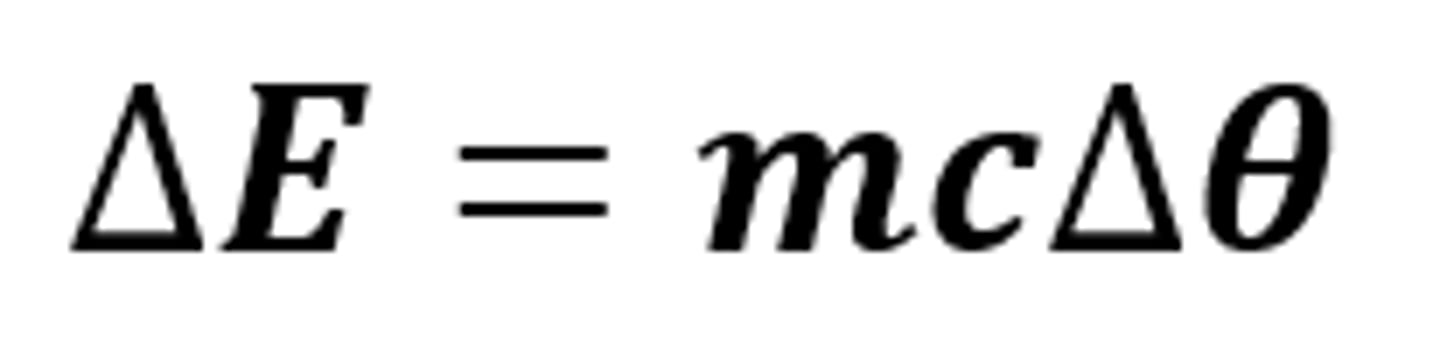
Joules per kilogram-degrees Celsius (J/kgºC)
The SI unit for specific heat capacity
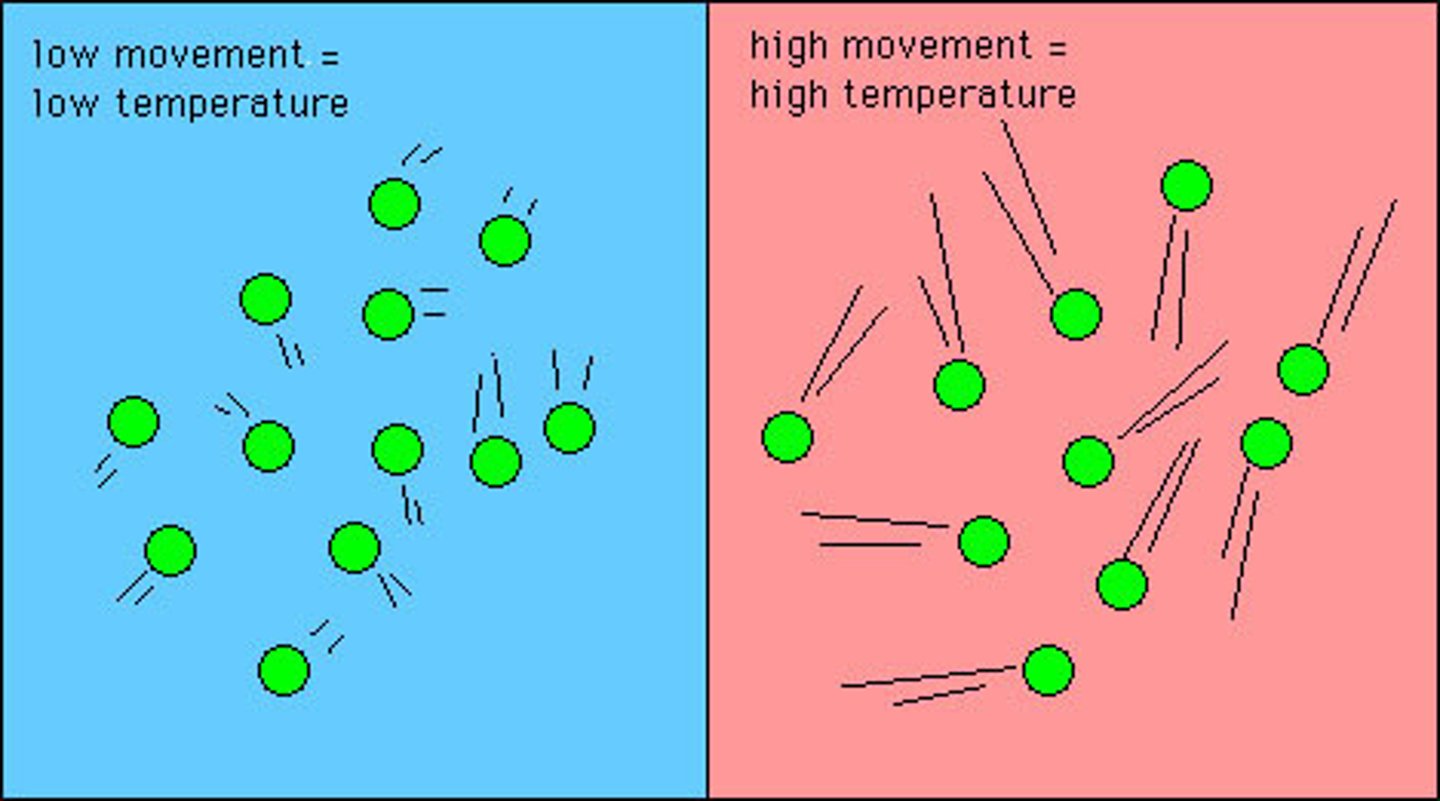
Temperature
A measure of the average kinetic energy of the particles in a substance
Specific heat capacity (SHC)
The energy required to raise the temperature of one kilogram (1 kg) of a substance by one degree Celsius (1 ºC)
ΔE
The symbol for the change in thermal energy
m
The symbol for mass
c
The symbol for specific heat capacity
Δθ
The symbol for change in temperature
Joules (J)
The SI unit for energy and work

Kilograms (kg)
The SI unit for mass

Degrees Celsius (ºC)
The SI unit for temperature

4200 J/kgºC
The specific heat capacity of water

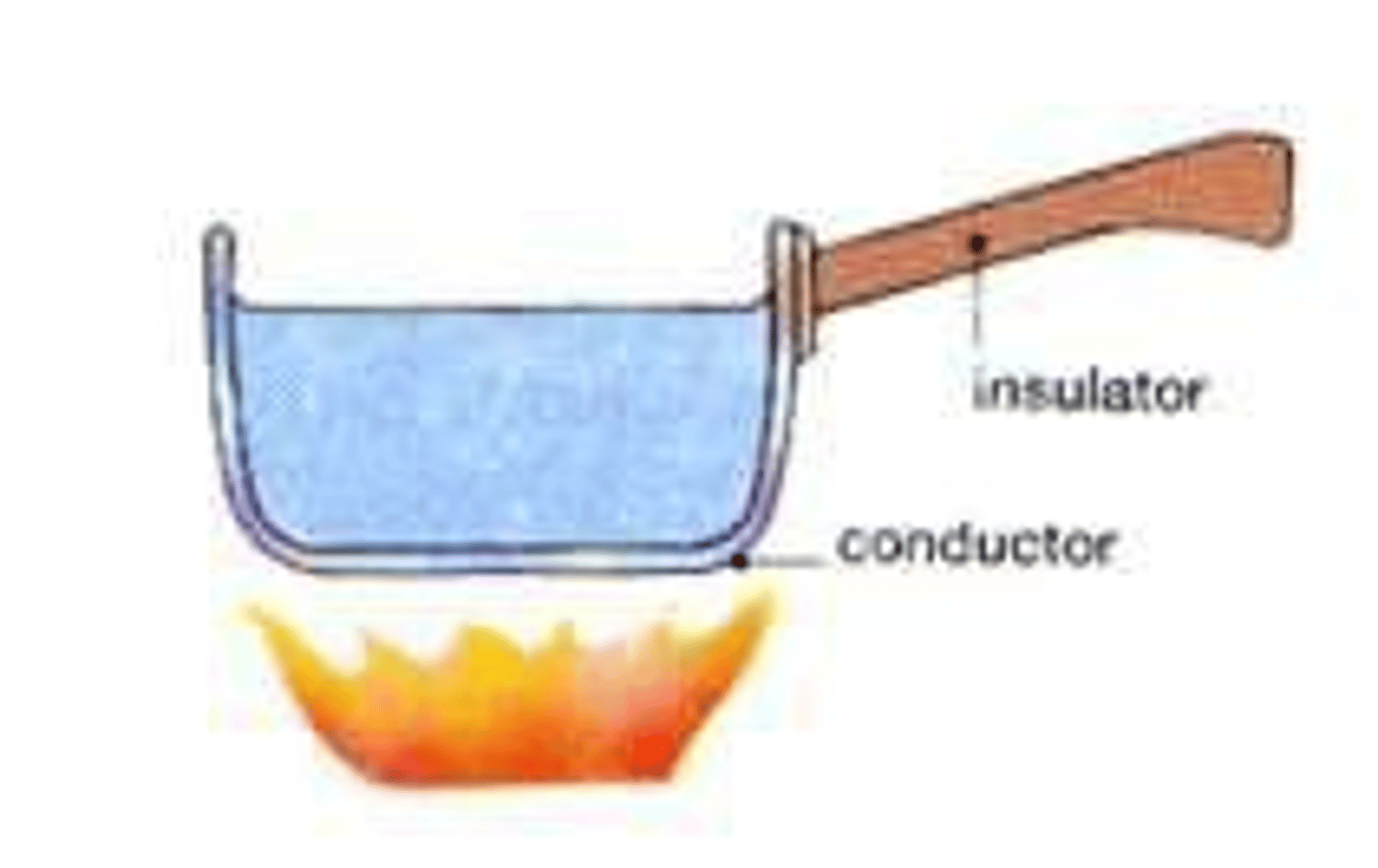
Thermal conductors
Materials with a low specific heat capacity so heat up and cool down quickly
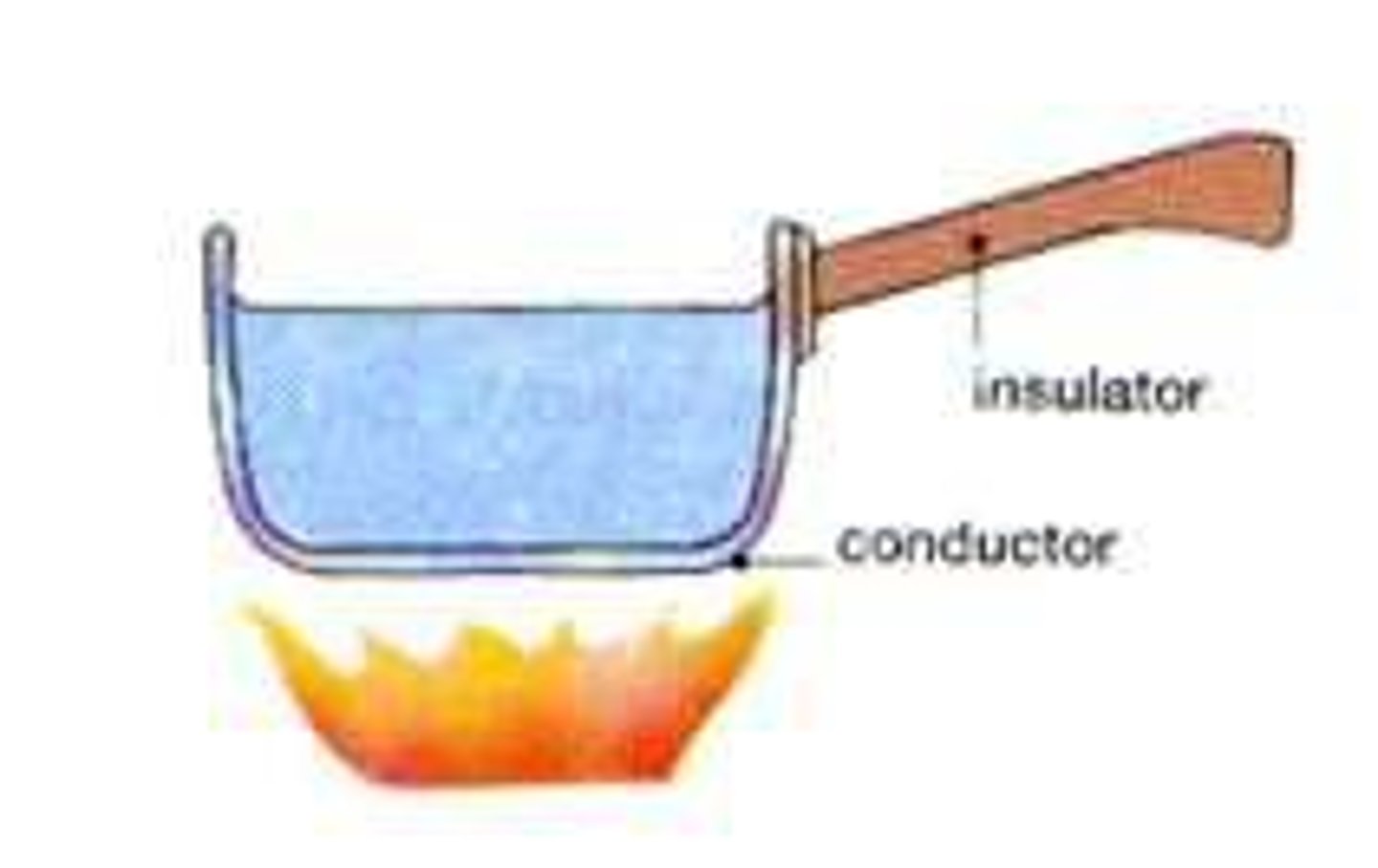
Thermal insulators
Materials with a high specific heat capacity so heat up and cool down slowly