Quantative Chemistry
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
What is a MOLE?
A MOLE is simply the relative atomic mass or relative formula mass of any substance expressed in grams.
What is Avogadro's constant?
A mole of any substance always contains the same number of atoms, molecules or ions.
What is Avogadro's number?
6.02 x 10 to the power of 23
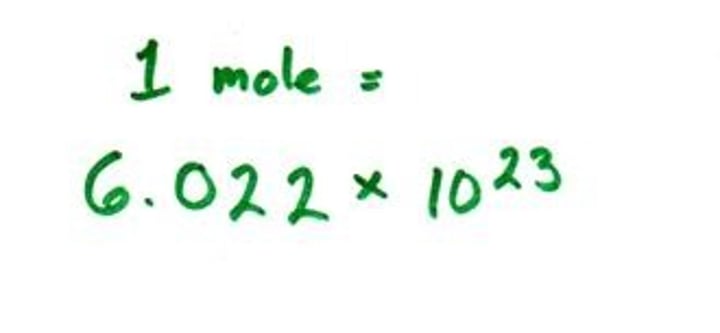
Number of moles=
mass/atomic mass
How can you tell the number of moles from a balanced symbol equation?
In a balanced symbol equation, the big numbers can also tell us how many moles of each substance will react or be formed.
What is % yield?
the amount of product you actually make as a percentage of the amount of product you should theoretically make.
What is the theoretical yield?
the maximum mass of a product expected from a reaction, using reacting masses.
What is the actual yield?
The actual yield is the mass of the product that is actually obtained from the real chemical reaction
Percentage yield equation
actual mass/theoretical mass x 100
Why is yield important?
- reduce waste
- less raw material used
- less energy for more product
- make more money
Reduced yield by:
- some product may be lost when it is transferred
- the reactions may form unexpected products
- the reaction in incomplete
- the reaction is reversible so may be incomplete
-some of the reactants may be impure
What is Titration?
a more accurate method to measure the exact volumes of acid and alkali needed to react with each other to make a neutral solution.
What is an end point?
The point at which the acid and the alkali have reacted completely
How do you judge when the end point is reached?
using an acid/base indicator
How do you improve the accuracy of a titration-
- the burette and pipette are washed with deionised water, followed by the appropriate solution before use.
- the burette is read at the bottom of the meniscus
-the conical flask is swirled throughout
-The acid is added drop wise near the end point
-the titration is repeated until concordant results
- only the concordant results are used to indicate the mean titre
Concentration equation
concentration= mass dissolved/ volume of solution
2nd concentration equation
no. of moles/ volume
Why is a volumetric flask more accurate for making up solutions?
Because it has a long thin neck so it is easier to read the scale at the meniscus. The bunged top and the shape of the flask itself, means it is easier to mix the solution by swirling. It measures to only one value so it is easier to read.
Economical effects of waste
- reactions unprofitable
-raw materials are expensive so shouldn't waste
-waste products expensive to dispose of responsibly
Environmental effects of waste
-by-products may be harmful to the environment
-waste materials have to be disposed of
Sustainability effects of waste
-resources used up quickly
-non-renewable materials will run out
What is R.T.P?
short for room temperature and pressure. This is taken as 20*C and 1 atm pressure
Equation with R.T.P
moles= volume/ 24 at RTP