Lecture 20 - Transplantation
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
65 Terms
MHC Class I Molecules
HLA-A, HLA-B, HLA-C
All nucleated cells
MHC Class II Molecules
HLA-DP, HLA-DQ, HLA-DR
APCs, few other cells
Major Histocompatibility Complex (MHC) Molecules
are cell-surface proteins essential for presenting antigens to T cells, which triggers an adaptive immune response
Haplotypes
inherited as a set
Most polymorphic genes….
elicit strong immune response
MHC genes are the most polymorphic genes in humans meaning they have many variants
Different MHC alleles can elicit strong immune responses
Autograft
Transplantation of your own tissue to yourself
No immune rejection because MHC molecules match exactly
Syngeneic Graft/Isograft
Transplant between genetically identical individuals (e.g., identical twins)
Minimal to no rejection due to identical MHC alleles
Allogeneic Graft
Transplant between two genetically non-identical people of the same species
Most common type in clinical practice
Strong immune response due to differences in MHC alleles
Xenograft
Transplant between different species (e.g., pig → human)
Very strong immune response
Strong response vs. MHC (huge MHC mismatch)
Highest rejection risk
Histocompatibility Antigens
MHC
Minor Histocompatibility Antigens (mHAs)
ABO blood group antigens
MHC Class I-Related Chain A (MICA) Antigens
Killer Immunoglobulin-Like Receptors (KIRs)
Histocompatibility Antigens - MHC
Sibling matches; Haplotypes inherited as set from each parent
HLA-identical
25% chance; both haplotypes shared
Haploidentical
50% chance; one haplotype shared
HLA-nonidentical
25% chance; no haplotypes shared
Minor histocompatibility antigens
Polymorphic non-MHC self-proteins
Normal human proteins that vary between individuals due to genetic differences
These differences make their peptides appear “foreign” to another person’s immune system
Why Minor histocompatibility antigens matter in transplants
Even when MHC is fully matched, the recipient’s T cells can still react to donor peptides derived from mHAs
Leads to slower, weaker rejection compared to mismatched MHC
mHA peptides are processed normally by donor cells and presented on MHC molecules to recipient T cells
Often presented on MHC Class I
Examples of Minor histocompatibility antigens
Some Y chromosome proteins
Some autosomal proteins
Why ABO Blood Group Compatibility in Transplantation is critical
A and B antigens are not only on RBCs — they are also expressed on endothelial cells of solid organs (kidney, heart, liver, etc.).
If a recipient has pre-existing IgM antibodies against donor ABO antigens → immediate immune attack
ABO Blood Group Compatibility in Transplantation - Mechanism of action
Recipient anti-A or anti-B antibodies:
Bind to donor A or B antigens
Activate complement system
Cause hyperacute rejection
Onset: minutes to hours
Leads to thrombosis, inflammation, and rapid graft destruction
Therefore:
Recipient and donor must be ABO identical or compatible for solid-organ transplantation.
Universal RBC Donor
Type O-
Lacks A, B, and Rh (D) antigens → least likely to be attacked by recipient antibodies
Universal RBC Recipient
Type AB+
Lacks antibodies to A, B, or Rh antigens → can receive RBCs from any type.
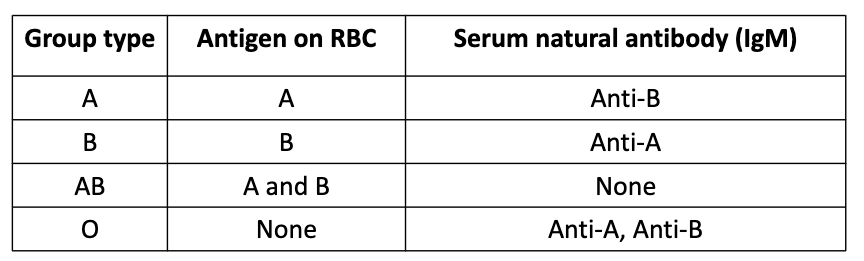
ABO Blood Group Chart
MHC Class I-Related Chain A (MICA) Antigens
Function / Immune Role
Involved in activating certain T cell responses (γδ T cells)
Expressed by various cells
Endothelial cells, Epithelial cells, DCs, others
Not expressed on lymphocytes
Highly polymorphic, with dozens of alleles
Killer Immunoglobulin-Like Receptors (KIRs)
Activating and inhibiting receptors on NK cells
Polymorphic
Alloreactive Response
Immune response against antigens from another individual of the same species.
In transplantation, this means the recipient’s immune system recognizing the donor’s MHC molecules as foreign
Alloreactive Response - If difference at MHC between donor/recipient
Response vs MHC molecule(s) on transplanted tissues
If the donor and recipient have different MHC alleles, the recipient’s T cells recognize the donor MHC proteins on the graft.
This leads to T cell activation → graft rejection.
Main Targets
MHC Class I response on most tissues
Both MHC I and II responses can occur
Memory cell vs non-self MHC Molecules
After the first exposure to donor MHC, the recipient develops memory T cells.
A second graft from the same donor triggers a secondary response to the graft (faster and stronger response)
This leads to an accelerated rejection
Why are MHC the main target?
due to the high frequency of T cells vs Non-self MHC molecules
Response to Alloantigens
Naïve alloreactive T cells become activated when they encounter alloantigens presented by APCs.
There are two main mechanisms of alloantigen presentation:
Direct allorecognition
Indirect allorecognition
Direct Allorecognition
The graft contains donor APCs
Donor APCs migrate from the graft to recipient lymph nodes and spleen via blood.
Recipient has a high frequency of T cells that can react to non-self MHC molecules.
Recipient T cells activated by donor APCs
TCR reacts directly to donor MHC molecules
Recipient TCR recognizes the donor MHC itself, with or without the bound peptide.
Activated T cells back to organ = destruction
Depletion of donor APCs from the graft delays rejection, because direct recognition is weakened.
Indirect allorecognition
Driven by recipient’s own APCs.
Process - Recipient APCs:
Take up allogeneic donor proteins
Non-self MHC and Minor histocompatibility antigens
Peptides are then processed and presented on self-MHC to recipient T cells
Effects on transplanted tissue
Antibody-mediated damage
Complement activation
ADCC (NK cell–mediated killing)
Direct cytotoxicity (T-cell killing)
Delayed-type hypersensitivity (Type IV reaction) inflammation
Acute Rejection to Allograft
Occurs within days to months after transplantation; as early as 10–13 days
T cell (CD8+, CD4+) and Antibody-driven
Memory cell develops
Acute Transplant Rejection - Pathology
Tissue and vessel damage
Many CD8⁺ T cells
MHC I expression on most cells = cytotoxicity
CD4+ T cells and Macrophages
Cytokines and delayed-type hypersensitivity
Antibodies to vessel walls
Complement, Inflammation, and transmural necrosis
Hyperacute Transplant Rejection
Occurs within minutes to hours after the graft’s blood supply is connected
Pre-existing alloantibodies = rapid rejection
Recipient already has antibodies against donor antigens vs.
MHC (HLA) antigens, Blood group antigens, and Endothelial cell antigens
Mechanism
Are complement-mediated
Antibodies bind/act on donor graft endothelium
Result
Clotting and complement
Damage and reduced blood flow
Enlarged graft; hemorrhaging and deoxygenation
Sources of these pre-exisiting allo-antibodies in Hyperacute rejection
Previous transplant
Prior blood transfusions
Response to paternal antigens in pregnancy
Prevention of Hyperacute rejection
ABO compatibility testing
Screening recipients for anti-HLA antibodies
Confirmed by Cross-matching of donor and recipient serum to ensure no reactive antibodies
Because of these steps, hyperacute rejection is now uncommon
Chronic Transplant Rejection
Late rejection of graft
Occurs months to years later after transplantation
Gradual loss of function
Difficult to detect and treat
Chronic Transplant Rejection Factors
Lengthy cold ischemia time for the graft
Ischemia–reperfusion injury during transplantation
Repeated, subclinical acute rejection events
These may go unnoticed but cumulatively damage the graft
Delayed-type hypersensitivity (DTH) responses to donor MHC molecules
Chronic Transplant Rejection Reaction - Chronic allograft vasculopathy
CD4+ T cells and alloantibodes
Espically in the heart and kidney
A progressive arteriosclerosis of graft vessels:
Leads to fibrosis, atrophy, and reduced blood flow
Liver: progressive loss of bile ducts
Lungs: bronchiole scarring (scarring and obstruction of bronchioles)
Graft-Versus-Host Disease (GVHD) Context: Hematopoietic Cell Transplants (HCTs)
Used to treat hematopoietic cancers
Patient’s own bone marrow is depleted (chemotherapy/radiation).
Repopulated immune response with donor cells
Graft-Versus-Host Disease (GVHD) (Complication HCTs)
Donor graft contains mature T cells
These donor T cells recognize recipient tissues as foreign
Severe inflammation is several tissues
Because the donor immune system is attacking the host → GVHD
Importance of HLA Matching
HLA matching is critical, especially for:
Allogeneic cell transplants
Sibling donors usually preferred (highest chance of MHC match)
Even with good HLA match, donor T cells can react to minor histocompatibility antigens
Benefits of Mature T cells in Hematopoietic cell transplants (HCT)
Reconstitute immunity quickly (helps fight infections)
Provide graft-versus-leukemia (GVL) effec
Donor T cells can kill cancer cells
GVHD Effects
“Cytokine Storm”
Massive activation of donor T cells
High cytokine release → widespread inflammation
GI track, skin, and liver
Often within 3 months
Later effects = Fibrosis of mucosa
Suppression of GVHD
Immunosuppressive therapy
Prior removal of mature T cells from stem cells
T cells that develop after transplant (from donor stem cells maturing in the host)
Become tolerant to recipient antigens
Lower risk of GVHD
Immunosuppressive Agents Purpose and Risks
Purpose
Inhibit rejection responses after transplantation
Essential for graft survival
Risks
Increased susceptibility to infection and cancer
Toxic side effects depending on the drug
Major categories of Immunosuppressive Agents
Corticosteroids
Calcneurin inhibitors
Antimetabolites
Monoclonal antibodies
Other Non-antibody drugs
Immunosuppressive Agents - Corticosteroids
Anti-inflammatory and immunosuppressive
Blocks cytokines, inflammatory molecules, and cell adhesion molecules
Immunosuppressive Agents - Calcneurin inhibitors
Blocks signaling for T cell proliferation and differentiation
Immunosuppressive Agents - Antimetabolites
Blocks lymphocyte maturation and destroys proliferating cells
Immunosuppressive Agents - Monoclonal Antibodies
Depletion of Mature T Cells (Anti-CD52)
Given at time of transplant
Removes mature T cells from circulation
Used also in bone marrow transplant to deplete donor mature T cells
Inhibits TCR Signaling (Anti-CD3)
Reduces activation by APCs
Anti-CD4, Anti-CD28, Anti-CD40L
Reduce T cell activation (Anti-CD25)
Blocks IL-2 signaling
Immunosuppressive Agents - Non-antibody drugs to target
Cell cycle
Translocation of nuclear factors
Differentiation cascades (in T and B cells)
Histocompatibility Testing - Mixed Lymphocytes
Purpose
Detects alloreactive donor T cells
Assesses the likelihood of T-cell–mediated rejection or GVHD
Especially important in hematopoietic cell transplantation
Cells Used
Donor lymphocytes (responding cells)
Recipient cells (stimulator cells)
Recipient T cells or APCs
Irradiated to prevent proliferation
Why Irradiate Recipient Cells?
Prevents recipient cells from dividing
Ensures that any observed response comes from donor T cells only
Mechanism
Donor T cells are mixed with irradiated recipient cells
Donor T cells recognize recipient alloantigens (non-self MHC)
This recognition triggers donor T-cell activation
Recognition of recipient allo-antigens by donor cells
Proliferation - CD4⁺ T cells respond mainly to MHC class II
Measured by increased DNA synthesis or cell division
Cytotoxicity -
CD8⁺ T cells recognize MHC class I
Kill recipient target cells
Histocompatibility Testing: HLA Typing
Determines HLA antigens or genes of a donor or recipient
Used to assess histocompatibility before transplantation
Complement-dependent cytotoxicity (CDC) test
Purpose
Determine HLA phenotype
Cells Used
Lymphocytes from the individual being typed
Cell type by HLA class
MHC Class I typing:
T cells and B cells (both express MHC I)
MHC Class II typing:
B cells only (express MHC II)
Reagents/Procedure
Antisera with known HLA specificities
Add reagent Complement
Add dye; taken in/enters dead cells only
Measure the proportion of dead cells using standard scale
Histocompatibility Testing: HLA Typing — Molecular Methods
Purpose
Determine HLA genotype
More precise than serologic (CDC) typing
PCR amplification of HLA genes
Specific HLA alleles and allele groups identified
PCR with Sequence-Specific Primers (PCR-SSP)
Principle
Uses panels of primers, each specific for a particular HLA allele or allele group
Perfect base-pair matching is required for amplification
Only reactions with matching primers → DNA amplification
Can ID HLA genotype based on primers that led to amplification
PCR with Sequence-Specific Oligonucleotide Probes (PCR-SSOP)
Principle
Single PCR reaction amplifies all relevant HLA genes
Amplified DNA is then tested with a panel of labeled DNA probes
Process
Each probe is specific for a particular HLA allele
Hybridization of probe to PCR product indicates presence of that allele
HLA genotype is then determined
Sequence-Based Typing (SBT)
Principle
Direct sequencing of HLA genes
Key Features
Considered the gold standard for HLA typing
Can identify new alleles
Histocompatibility Testing: HLA Antibody Screening
Purpose
Detect antibodies against HLA antigens in patient serum
Critical for transplant planning and monitoring
Informs about potential donors
When & Why Testing Is Done Patients on Transplant Waiting Lists
Patients on waiting list tested periodically
Transplant Recipients
Tested periodically to:
Assess rejection
Monitor effectiveness of anti-rejection therapy
Complement-dependent cytotoxicity test
Uses panels of lymphocytes with known HLA antigens
Add:
Patient serum
Complement
Vital dye (enters dead cells)
Interpretation
Antibody binding → complement activation → cell death
Cell death indicates patient serum contains Abs to that HLA antigen
ELISA (Indirect)
HLA antigens coated onto microtiter wells
Add patient serum
Add enzyme-labeled secondary antibody
Detection
Color change indicates presence of anti-HLA antibodies
Flow Cytometry
Beads coated with HLA antigens
Incubated with patient serum
Add fluorescently labeled secondary antibody
Multiplex Bead Array
Allows detection of antibodies against many HLA antigens in one tube