chem battery
Describe a voltaic cell
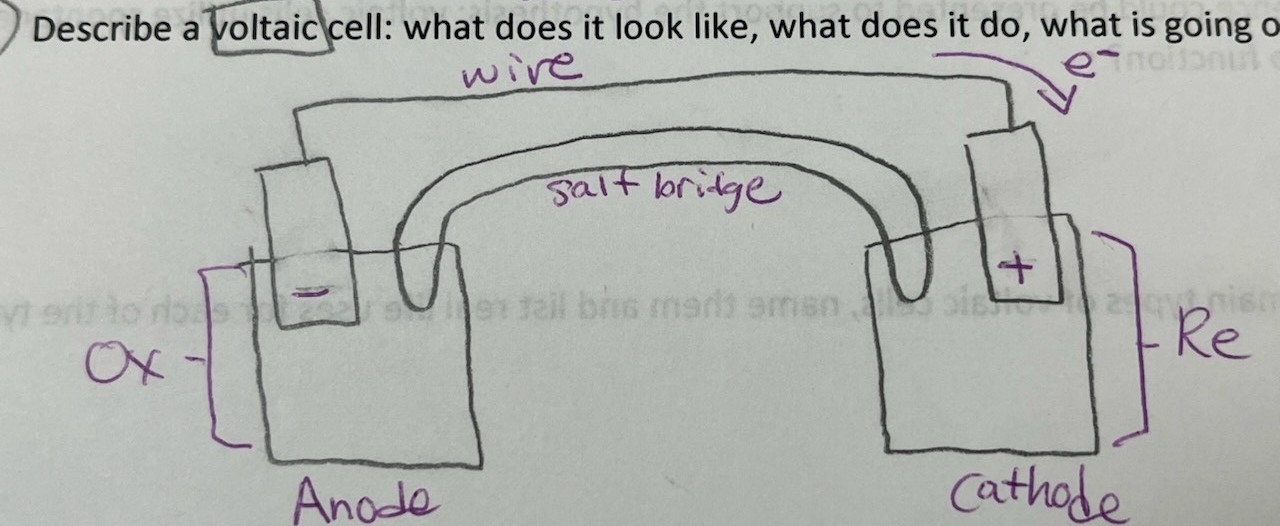
wire, salt bridge, oxidation, reduction, (e-) ----> , anode (-), cathode (+)
Describe an electrolytic cell
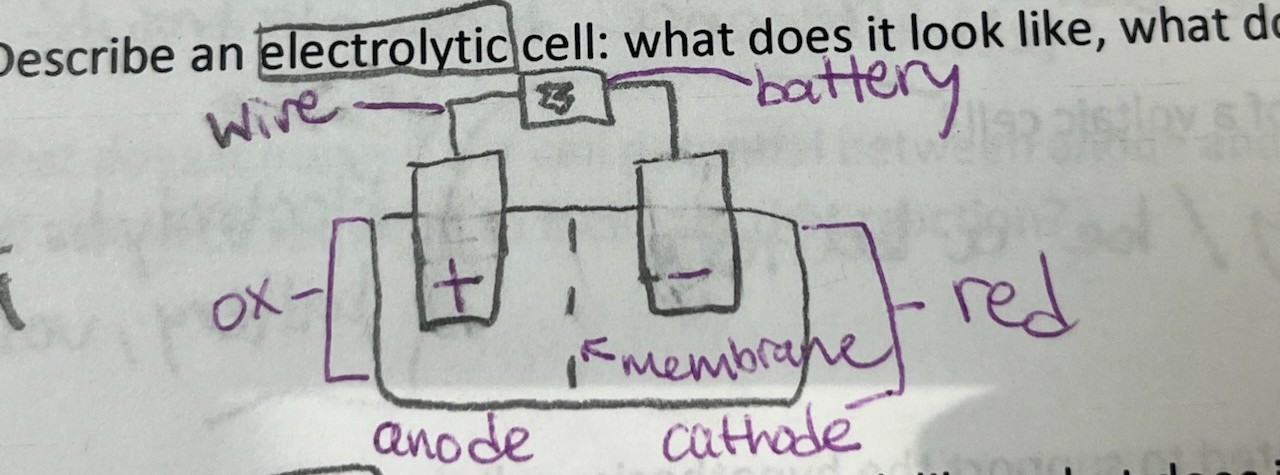
wire, battery, oxidation, reduction, membrane, anode (+), cathode (-)
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
Describe a voltaic cell
wire, salt bridge, oxidation, reduction, (e-) ----> , anode (-), cathode (+)

Describe an electrolytic cell
wire, battery, oxidation, reduction, membrane, anode (+), cathode (-)

what is the primary purpose of electrolytic cells?
to force spontaneous reactions to occur/ break compounds into elements w/ electricity
what is the primary purpose of voltaic cells?
produce electricity
difference between electrolytic and voltaic
electrolytic cells need a battery, voltaic dont
purpose of salt bridge in voltaic cell
over time anode becomes (+) and cathode becomes (
cell potential equation
cathode
What does it mean if the cell potential between anode and cathode is positive? What type of cell would be required to facilitate that reaction?
If answer is positive, battery works and reaction is spontaneous. You would need a voltaic cell
- What does it mean if the cell potential between anode and cathode is negative? What type of cell would be required to facilitate that reaction?
If answer is negative, not a battery and nonspontaneous. electrolytic cell is required
practice cell potential on sheet
.
which would not be a battery (cell potential)
all the negative answers
which would be the better battery when given two positives
the bigger number
normally, you would need two metals to make a battery, however this is not the case for lead. Why?
there is two of them
why cant you make a pure lithium battery?
there is only one