Water Molecule Structures
1/18
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
Non-Polar Covalent
Share electrons equally
Non-Polar Covalent
Share electrons unequally
Hydrogen Bond
Attraction between two polar molecules
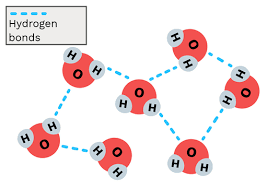
Polar Molecule
Molecule where one end is + and the other -
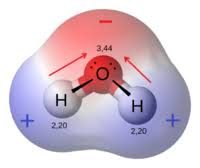
How are H2O molecules shared?
Polar covalent bonding
Are water molecules polar or non-polar?
Polar
Are hydrogen atoms + or -
Partially positive
Are Oxygen atoms + or -
Partially negative
Cohesion
Water mol. sticking to each other through hydrogen bonding
Adhesion
Water molecules hydrogen bonding to another kind on molecule
Why is water a universal solvent?
It is very abundant
Polarity
(+/-) ends allow it to interact with anything that had a charge
Hydrophilic
Polar and Ionic substances are…
Hydrophobic
Non-Polar molecules are….
Buoyancy
An upward force applied to an object immersed in a fluid
Viscosity
Measure of a fluids tendency to flow
Thermal Conductivity
A measure of a materials ability to move heat
Specific Heat capacity
The quantity needed to raise the temp of a chemical per unit mass
Carbon always forms…
4 covalent bonds
Xylem
vascular tissue in plants that transports water from roots to leaves