2.4 - Transition Metals
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
Give 2 properties of Transition Metals
Transition metals occupy the d-block
Catalytic
Give 2 examples of Transition metals acting as a Catalyst
Platinum in fuel cell
Nickel in nitrile reduction to amines
What are the colours of CuSO4 in different states? (when light shines on it)
Why
Solid = White
Liquid = Cyan
Red light is absorbed so the complimentary colours show
How do we see a compound’s colour when light is cast on it?
Lights different wavelength of light shows the corresponding colours which have the exact amount of energy to excite electrons to the next orbital (given it has space for it)
What is the usual order of Subshells for Transition Metals?
2 anomalies + configuration (why)
3d 4s:
Cr = [Ar] 4s13d5 → 3d is half filled to similar energy level to 4s, so the electron occupies it
Cu = [Ar] 4s13d10
What does this effect in terms of ionisation?
When being ionised for Cu + Cr, the 4s s lost first or gains the electron
Why do Transition metals form coloured compounds?
Range of metals
Transition metals are the only ions that are stable, whilst having partially filled d-orbitals → excitation of electrons to the partially filled orbitals and their de-excitation gives colour:
Ti → Cu
Complex Ions
Complex Ions
What is a Ligand? (3 examples)
A particle with a lone pair that datively covalently bonds to a transition metal ion:
H2Ö
N̈H3
:Cl-
Why do Complex Ions with the same Metal have different co-ordinate numbers?
Ligands, for example Cl, are greater in size than other ligands, like NH3, can only occupy space as a tetrahedral
What is the priority for a Complex Ions when writing general formula?
Example = Amino,aqua,di-chloro platinum (II)
Neutral
Positive
Negative
[Pt(NH3)(H2O)(Cl)2]0
Name the following Complex ions:
[V(H2O)6]3+
[Co(NH3)6]2+
[Co(Cl)4]2-
[Co(H2O)3(NH3)3]2+
Hexa Aqua Vanadium(III)
Hexa Ammine Cobalt (II)
Tetra Chloro Cobaltate (II) → “-ate” for negative complex ions
Triammine,Triaqua Cobalt (II)
Which 3 metals have Square Planar complex ions - no matter what ligands are attached to them?
What are the shapes of the rest
Platinum, Nickel, Palladium:
All other metals have Octahedral shapes → square planar for large ligands
When is a Square Planar complex ion:
Cis
Trans
Cis = When identical ligands are on the same side (Left or right)
Trans = When identical ligands are on the opposite side
When is Transplatin used?
Trans platin is more stable than Cisplatin, so it’s used when transporting → then it is converted to Cisplatin for cancer treatment
When is an Octahedral Complex ion:
Cis
Trans
Cis = When identical ligands are 90o from eachother (basically when 2 identical ligands are separated by one different ligand)
Trans = When identical ligands are on opposite sides of eachother
What is:
Polychromatic light
Monochromatic light
Polychromatic light = Contains mixture of wavelengths of light of different colours
Monochromatic light = Contains one wavelength of light of the same colour
What are the gaps between orbitals equivalent to?
Frequency of light needed to excite electrons
Why are most compounds colourless?
Most compounds energy level split is too larger so light doesn’t hold enough energy to excite electrons
Why do Complex ions form coloured compounds?
When ligands datively covalently bond to ions, the metal ions d-orbitals split equally. The gap is small so wavelengths of light that are shone onto a sample can excite electrons from the lower half of the orbital to the upper half, when electrons de-excite they emit wavelengths of light with a corresponding colour
Why do few Complex ions not form coloured compounds?
Example of a metal that doesn’t
When the d-orbital is full, there is no space for a lower electron to excite + occupy the orbital, so when light is shone, no colour is transmitted:
Zinc
What is the relationship between Energy level gap of orbitals and frequency?
Include in terms of colour
Direct proportion:
Higher frequency = Blue-er
Lower frequency = Redd-er
What are the 2 formulae for the Energy between orbitals?
E = hf
E = hc/λ
h → planck’s constant = 6.63 × 10-34
What is the relationship of Oxidation state and Frequency?
(+ colour observed)
Direct proportion = As oxidation number increases, it absorbs a larger frequency of light:
Colour observed is less blue
(vice versa)
Order the following ligands in terms of D-Orbital splitting, Absorbed Frequency, Energy gap between orbitals, Colour:
NH3
H2O
Cl
> D - Splitting / Frequency + Energy change (increasing):
Cl, H2O, NH3
> Colour (how blue it is):
NH3, H2O, Cl
Order the following Co-ordination numbers in terms of D-Orbital splitting, Energy gap between orbitals, Colour:
Octahedral (C.No = 6)
Square Planar (C.No = 4)
Tetrahedral (C.No = 4)
> D - Splitting / Frequency + Energy change (increasing):
Tetrahedral, Square Planar, Octahedral
> Colour (how blue it is):
Octahedral, Square Planar, Tetrahedral
Explain the effect of Complex ion’s shape on its colour?
What is the additonal factor
Ligands effects the geometry of molecules so effect the ions D-splitting:
Type of metal ion
Colorimetry
Colorimetry
What is Colorimetry?
2 advantages
Use of light source + colour filter, shining the light at the sample with a complimenting colour to the light, then measuring the amount of light absorbed by the solution:
Quick process
Doesn’t require large sample
Give the 3 step method to carry out Colorimetry
We compare our sample absorption to know concentration + their absorption
Plot a graph of these values
Extrapolate the absorption from STEP 1 to the graph to find the corresponding concentration
What are the axis’ for a Calibration curve?
x-axis = Concentration
y-axis = Absorption
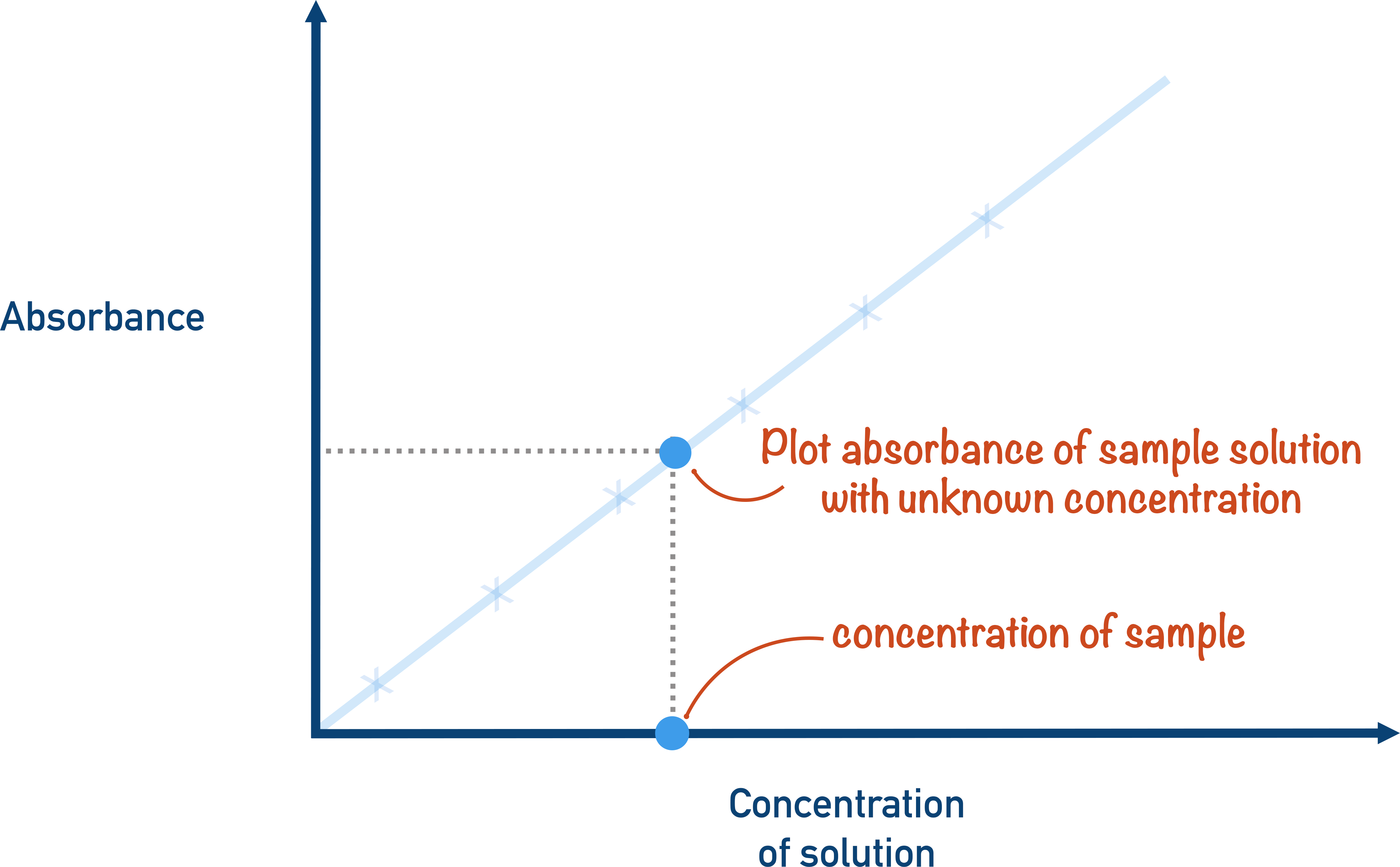
Why is their a plateau on Calibration curve?
Absorption not proportional to concentration
What is Complete ligand substitution?
Where all ligands are replaced
What is Incomplete ligand substitution?
Where a few ligands are replaced
What are the products of:
[Co(H2O)6]2+ + 4Cl-
Why is this?
Entropy of this reaction
[Co(H2O)6]2+ + 4Cl- → [Co(Cl)4]2- + 6H2O
Cl is too large so can replace all 6 water ligands with 4 Cl
High entropy since more products than reactants
What is a Bidentate Ligand? (co-ordinate number)
Singular Ligands that form 2 dative covalent bonds to a complex ion:
1 bidentate ligand = 2 co-ordinate number
What is prefix for the following Complex ions:
2 bidentates
3 bidentates
4 bidentates
2 bidentates = Bis
3 bidentates = Tris
4 bidentates = Tetrakis
What is the structure of the “en” ligand?
Type of ligand
Charge
C2H4(NH2)2
Bidentate
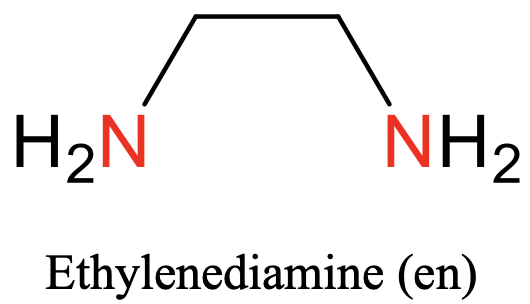
Charge = 0
What is the structure of the Oxylate ligand?
Type of ligand
Charge
C2O42-
Bidentate
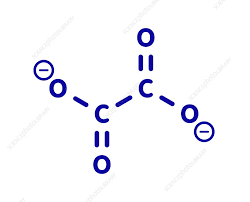
Charge = -2
What type of Isomerism do Complex ions observe?
Exceptions
Optical Isomers → enantiomers of eachother:
Linear/Square planar ions
What is the Chelate effect?
The replacement of monodentate ligands to multidentate ligands, such that entropy increases
Comment + Explain on the following about the Chelate effect:
Entropy
Feasibility
Entropy increases as the number of reactants < products. So entropy of forward reaction is high - meaning reaction is likely to occur + since enthalpy = 0 → reaction is always feasible
What is EDTA?
Number of lone pairs, atoms and charge
Entropy → its effect
A multidentate ligands with:
4O:- and 2N: so one EDTA replaces 6 ligands, -4 charge
High entropy of 2 reactants to 7 products → quickly reacts
What is the use of EDTA? (explain)
Prevention of lead poisoning in body, large entropy so feast reaction which quickly halts the effect of Pb poison in the body by forming harmless complex ions with Pb
What is Haem?
Describe structure
Bonds formed
What is bonds with
Haem is an Fe(II) complex with multidentate ligand that can form 4 dative covalent bonds:
Bonds with protein globin
Forms Haemoglobin complex
What does the Haemoglobin complex can do? (use)
Forms co-ordinate bonds with O2 + H2O ligands which are easily formed + broken:
Respiration
Explain how Haemoglobin work with Oxygen, Water and Carbon Dioxide
Haem forms weak co-ordinate bonds with oxygen, water and carbon dioxide so easily transports to + from muscles. Don’t form stable bonds with Fe2+
Explain how CO poisoning with Haemoglobin occur
CO forms stable bonds w/ Haem so decreased number of useable Haem for respiration meaning no Oxygen absorption, and increased Carbon Dioxide + CO in blood leading to death