Behavioral Neuroscience Exam 3
1/133
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
134 Terms
Gender
The behaviors and attitudes that a given culture considers to be masculine, feminine, or an alternative
Sex
A trait that determines whether a sexually reproducing organism produces male or female gametes
Gonadal hormones (e.g., testosterone and estradiol)
Mediate early changes in the developing brain to assure that activation of the same circuits by the same hormones in adulthood will produce the appropriate reproductive behaviors
Reproductive behavior
Behaviors for attracting and finding a mate, copulation for exchange of gametes (i.e., sperm, ovum), care and nurturing of offspring on the brain during development
Most of these functions are differentiated in males and females, and due to genetic and hormonal effects on the brain during development
Rehash of sex ed
23 chromosomes pairs in humans; 22 autosomes and 1 sex chromosome – X or Y
a. XY pair = male
b. XX pair = female
Sex hormones are secreted by gonads (testes, ovaries), and they have a steroid structure, hence they are called gonadal steroids or gonadal hormones
Androgens
Gonadal steroids synthesized in testes
Testosterone: An important androgen
Estrogens
Gonadal steroids in females synthesized in ovaries
Estradiol: An important androgen
Sex determination
Process by which the decision is made for a fetus to develop as a male or a female – if the sperm that enters the egg has a Y chromosome, the offspring is male; if an X chromosome, the offspring is female
There is a single gene on the Y chromosome called SRY (sex-determining region of the Y chromosome)
SRY (sex-determining region of the Y)
Directs the formation of testes from the “bipotential” gonad (gonadal precursor to either testes or ovaries), everything else follows from this initial step!
In the absence of SRY (or if there is a loss-of-function mutation in SRY), the default developmental program is toward the female, which means an XY female may occur in rare instances

Sexual differentiation
Process by which individuals develop either male or female bodies and behaviors
After sex determination, hormones secreted by gonads, mainly the testes, direct sexual differentiation (remember if no testes form, then one becomes female)
Embryos have early tissues for both male and female structures
The wolffian and the müllerian ducts connect the indifferent gonads to the body wall
Müllerian ducts
Develop into the female reproductive organs (oviducts, uterus, vagina), most of the wolffian system degenerates
Wolffian ducts
Develop into the male reproductive organs (epididymis, vas deferens, seminal vesicles), and the müllerian system shrinks
Sexual differentiation of the brain occurs during a sensitive period
Steroids have an organizational effect only when present during a sensitive period in early development
Depending on the species and the behavior, it may be before birth or just afterwards, in the neonatal period
In mammals, puberty can be viewed as a second sensitive period
Organizational/activational hypothesis (OAH) — William Young
A framework used to describe the two sensitive periods for gonadal steroid influences in sexual differentiation
“Organizational”
During gestation, the onset of testosterone secretion in males defines the sensitive period for sexual differentiation in the brain – start of masculinization
The end of this first sensitive period is defined as when females are no longer sensitive to the effects of androgens
“Activational”
Later surge of gonadal hormones in puberty and beyond serves to activate the previously organized brain networks
Note that this stage may be constrained by previous events
Rodent sex
In rats, females ovulate, or release eggs, every 4-5 days
The female displays proceptive behaviors, and adopts an arched-based posture called lordosis, allowing the male to mount the female and to engage in intromission (i.e., active process of inserting the penis into the vagina)
The male and female then orchestrate seven to nine intromissions
Only after repeated intromissions will the female’s brain cause release of hormones to support pregnancy
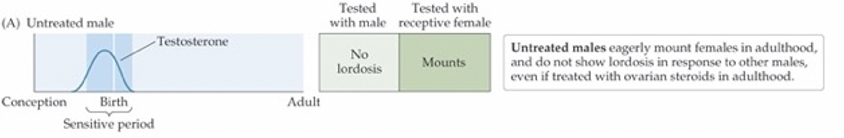
OAH — W.C. Young’s famous experiments in 1950s
One steroid signal—testosterone—masculinizes the body, the brain, and behavior, all during gestation
If androgen isn’t present, the nervous system will organize itself in a feminine fashion
Full masculine behavior requires testosterone during development and adulthood

Sexual dimorphism
Males and females have marked sex differences in appearance (sexual dimorphism also occurs in the brain)
In rats, a subregion in the pre-optic area (POA) of the hypothalamus is larger in many males relative to females
Sexually dimorphic nucleus of the POA (SDN-POA)
Lesions in this area disrupt ovulatory and copulatory behaviors in females and males, respectively
4 key CNS structures altered by organizing effects of gonadal steroids
1. Sexually dimorphic nucleus of the preoptic area (SDN-POA)
2. Anteroventral periventricular nucleus of the preoptic area (AVPV-POA)
3. Principal nucleus of the bed nuclei of the stria terminalis (pBST)
4. Spinal cord, nucleus bulbocavernosus (SNB)
These brain regions are generally larger in the male rather than female brain, and results from hormonal effects on cell death/survival during brain development
Gonadal steroids modulate brain regions not directly involved in reproduction
There are a variety of small but consistent brain differences in males and females
Hippocampus and amygdala show some differences that affect: a) emotionally, b) aspects or learning and memory, c) stress responsiveness, d) affiliative behaviors
Yet these are minor as compared to effects seen in POA and BST regions that drive sex-specific reproductive behaviors
These additional non-reproductive sex differences in brain function are also more subject to modification by experience and environmental factors
Homosexual behavior
The third interstitial nucleus of the anterior hypothalamus (INAH-3), is in the POA
INAH-3 was larger in men than in women, and larger in heterosexual men than in homosexual men
It is not clear if the size difference is a result or a cause of becoming gay
And it is important to highlight that these differences are not thought to be due to any clear organizing effects of androgens during gestation
Homeostasis
Regulation of biological processes that keep certain body variables within a fixed range
Note that this concept is more broadly evident in nervous system than just autonomic
Set point
Refers to a single value that the body works to maintain
E.g., levels of water, oxygen, glucose, sodium chloride, protein, fat and acidity in the body
Negative feedback
Processes that reduce discrepancies from the set point
Food and energy regulation
All the energy we need to move, think, breathe, and maintain body temperature is through breaking of chemical bonds in complex molecules down to smaller ones
33% of energy in food is lost to digestion
55% of energy is consumed by basal metabolism
12% is used for active behavioral processes
Food and energy regulation — steps
First step: Digestive system breaks down food for energy utilization/storage and nutrient absorption
2 pancreatic hormones
a. Insulin: Promotes glucose uptake into cells; promotes conversion of glucose to glycogen – a more storage-friendly carbohydrate — released in anticipation of and after a meal to promote glucose utilization
b. Glucagon: Increases blood levels of glucose; mediates breakdown of glycogen back to glucose
Without insulin, cells in the body (except in the brain, which actively transport glucose across the BBB) are not able to bring glucose into cells
Glycogen is a stored form of glucose (sugar) in your liver and muscles, while insulin is a hormone that tells your cells to use or store this glucose
Diabetes mellitus
Insulin levels remain constantly low, so blood glucose levels stay elevated
a. People eat more than normal, but excrete the glucose unused and lose weight
Type 1: Juveniles onset diabetes; pancreas stops producing insulin
Type 2: Adult onset; associated with obesity; consequence of reduced sensitivity to insulin
Classic lesion studies
Classic lesion studies first implicated the hypothalamus in control of appetite
Bilateral lesions of the ventromedial hypothalamus (VMH) could cause obesity in rats (i.e., hyperphagia)
Bilateral lesions in the lateral hypothalamus (LH) had the opposite effect (i.e., aphagia)
Arcuate nucleus (of the hypothalamus)
Appetite control center that is governed by a variety of circulating hormones in the periphery (e.g., insulin, leptin, ghrelin, cholecystokinin)
Information from all parts of the body regarding hunger impinge onto two kinds of cells in the Arcuate nucleus of the hypothalamus
1. Neurons sensitive to hunger signals (i.e., appetite-enhancing) -- NPY/AgRP (neuropeptide Y/Agouti-related protein) neurons
2. Neurons sensitive to satiety signals (i.e., appetite-suppressing) -- POMC/CART (pro-opiomelanocortin/cocaine- and amphetamine-related transcript) neurons
Leptin
Produced by the body’s fat cells when amount of fat stored reaches a certain level – when levels are low it signals to the Arcuate to increase appetite
Leptin was discovered from transgenic mice with defect in gene encoding leptin
Low levels increase hunger, although high levels do not necessarily decrease hunger
Some obese individuals show evidence of reduced leptin sensitivity, and leptin treatment reduces obesity in these folks
Ghrelin
Released into the bloodstream by endocrine cells in the stomach
Circulating levels of ghrelin rise during fasting and immediately drop upon eating
Treating rats/humans with ghrelin produces a rapid increase in appetite
PYY3-36
Released into the bloodstream from the gut
Its effects are opposite of ghrelin: levels are low during fasting, and rise right after eating
PYY3-36 is associated with feelings of satiety – effects of PYY are somewhat like leptin (i.e., satiety-related, hunger inhibiting)
NPY/AgRP neurons
Have appetite stimulating effects
Inhibit POMC/CART cells
Activate paraventricular hypothalamus (PVH)
Activate lateral hypothalamus (LH)
POMC/CART neurons
Have appetite suppressing effects
Inhibit MPY/AgRP neurons
Inhibit LH
Nucleus of the solitary tract (NTS)
Part of a central pathway for feeding behavior
Appetite-stimulating effects of PVH and LH are relayed to, and integrated in, the nucleus of the solitary tract (NTS)
NTS receives signals, such as stomach distention, change in glucose levels in the liver, etc., from the periphery via incoming vagus nerve
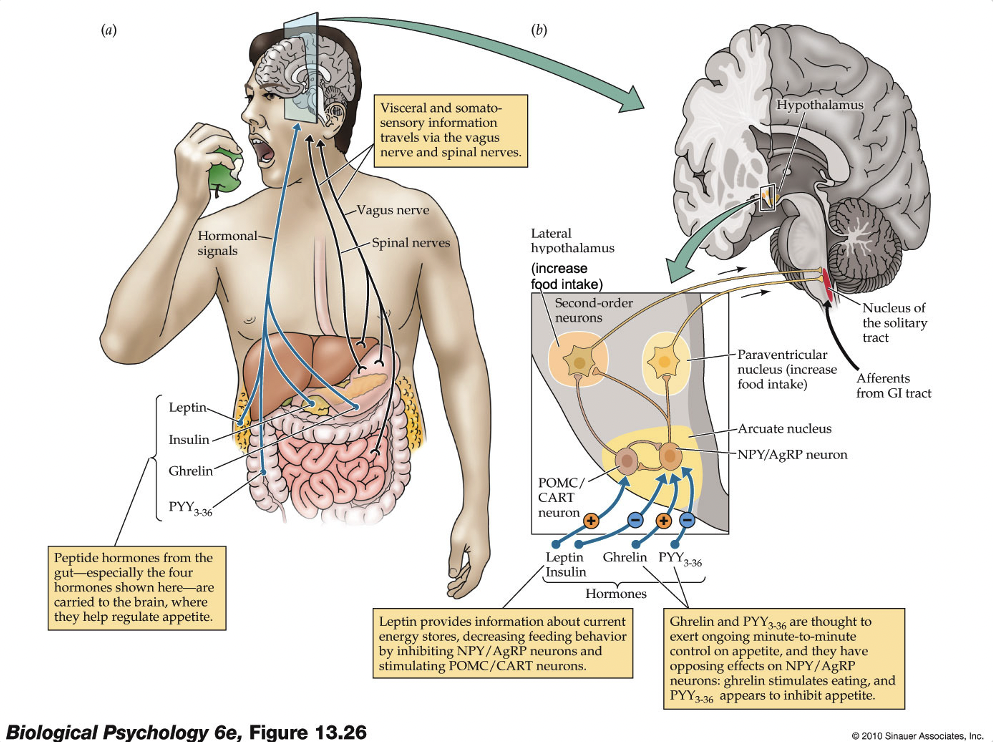
Cholecystokinin (CCK)
Another peripheral signal that is released by the gut after ingestion of food high in protein or fat
CCK release is associated with feelings of satiety
CCK activates receptors in the vagus nerve, that ascends to the NTS, to signal satiety by inhibiting NPY/AgRP and exciting POMC/CART neurons
(Drugs like Ozempic act on this NTS system)
Biological rhythms
Ultradian rhythms: Frequency > once/day (e.g., rest-activity cycle in humans is 90 min
Infradian rhythms: Frequency < once/day (e.g., menstrual cycle (primates) or estrous cycle (other mammals)
Some animals generate endogenous circannual rhythms, internal mechanisms that operate on an annual or yearly cycle
E.g., bird migratory patterns, animals storing food for the winter, hibernation
Why do we need a circadian rhythm?
The purpose of the circadian rhythm is to keep our internal working in phase with the outside world
Human circadian clock generates a rhythm slightly longer than 24 hours when it has no external cue to set it
**ALL LIFE ON EARTH HAS A BIOLOGICAL MECHANISM FOR TIME KEEPING
Free-running rhythm
A rhythm that occurs when no stimulus resets it; it is still rhythmic, but not phase-locked with day length
Phase shift
Shift of activity due to a shift in a synchronizing stimulus (e.g., changing time zones during airline travel)
Zeitgeber
Term used to describe any stimulus that entrains the circadian rhythm to the earth’s 24 light/dark cycle, light is the main one
Exercise, noise, meals, and temperature have been speculated to also act as zeitgebers
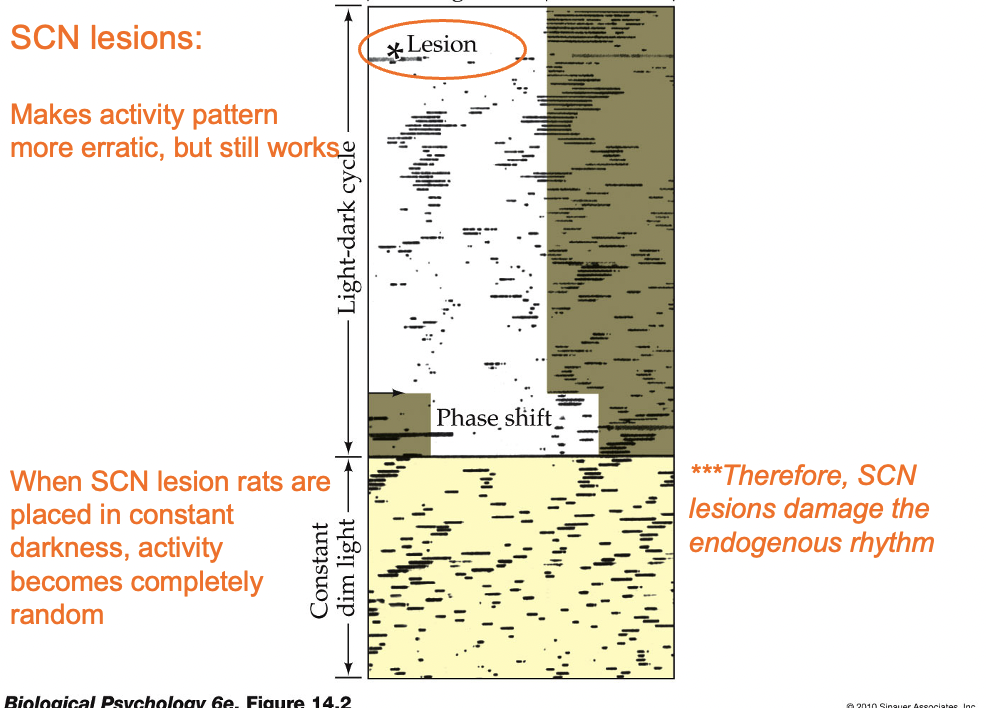
Suprachiasmatic nucleus (SCN)
Part of the hypothalamus and the main control center of the circadian rhythm
a. Located dorsal to the optic chiasm
b. Damage to the SCN results in less consistent body rhythms that are no longer synchronized to environmental patterns of light and dark
SCN sends information to hypothalamic nuclei (and indirectly, to the pineal gland) to modulate body temperature and production of hormones
Resetting the SCN
Light resets the SCN via a small branch of the optic nerve known as the retinohypothalamic tract, that travels directly from the retina to the SCN
The retinohypothalamic tract comes from a special population of ganglion cells that have their own photopigment called melanopsin – these cells respond directly to light and do not require any input from rods or cones
How does a cell “know” how to generate such a rhythm?
Early work with drosophila pointed to a gene called per (for “period”)
From there, the mechanisms underlying SCN circadian rhythms in mammals were eventually discovered
Melatonin and pineal gland
The SCN regulates waking and sleeping by controlling activity levels in other areas of the brain
The SCN regulates the pineal gland, an endocrine gland located posterior to the thalamus
The pineal gland secretes melatonin in the evening, which has mild sleep-evoking effects
Jet lag
Refers to the disruption of the circadian rhythms due to crossing time zones – stems from a mismatch of the internal circadian clock and external time
Characterized by sleepiness during the day, sleeplessness at night, and impaired concentration
Traveling west “phase-delays” our circadian rhythm
Traveling east “phase-advances” our circadian rhythms
Electroencephalograph (EEG)
Measures brain activity from noninvasive electrodes placed on the scalp
Allowed researchers to discover that there are various stages of sleep
The general trend of EEG changes from wake to sleep involve a shift from low amplitude, high frequency toward high amplitude, low frequency pattern
Alpha waves
Present when one begins a state of relaxation
Stage 1 sleep
When sleep has just begun – the EEG is dominated by irregular, jagged, low voltage waves, brain activity begins to decline
Stage 2 sleep
Sleep spindles: 12-14 Hz during a burst that lasts at least half a second
K-complex: A sharp high-amplitude negative wave followed by a smaller, slower positive wave
Stage 3 and stage 4
Constitute slow wave sleep (SWS) and are characterized by:
EEG recording of slow, large amplitude delta waves
Slowing of heart rate, breathing rate, and brain activity
Highly synchronized neuronal activity
Rapid eye movement (REM)
Also known as paradoxical sleep
EEG waves are irregular, low-voltage and fast (i.e., like waking)
Postural muscles of the body are more relaxed than other stages
Non-REM sleep (NREM)
Stages other than REM
When one falls asleep, they progress through stages 1, 2, 3, and 4 in sequential order
After about an hour, the person begins to cycle back through the stages from stage 4 to stages 3 and 2 and then REM
The sequence repeats with each cycle lasting approximately 90 minutes
Cerveau isolé (“isolated cerebrum”)
Transection through the mesencephalon; animal shows constant unresponsiveness and signs of continuous SWS
Encéphale isolé ("isolated brain")
Animal with transection at level of medulla; shows normal responsiveness and sleep-wake patterns
Classic studies suggested that SWS is produced by the forebrain (Bremer, 1935)
Bremer interpreted these observations to mean that the cortex must receive sensory afferent information (i.e., via the cranial nerves) for wakefulness
Morruzi and Magoun (1952) later showed that this was wrong, and that an “activating system” in the midbrain actively drives wakefulness
This became known as the ascending reticular activating system (ARAS)
ARAS (ascending reticular activating system)
A collection of anatomical structures with different functions, but there are “wake-maintaining” circuits in this region
We no longer think of the ARAS as driving wakefulness, our modern conceptions is of 4 major systems that interact to mediate states of arousal
Forebrain system
That can display slow wave sleep (SWS) by itself (a la Bremer)
A region called the basal forebrain promotes SWS by releasing GABA into the tuberomammillary nucleus in the hypothalamus. Electrical stimulation of the basal forebrain makes animals sleepy, while lesions of this region induce insomnia. Transections that create an isolated forebrain result in constant SWS.
Stimulation of the preoptic area (POA) produces sleepiness, whereas lesions produce insomnia
Basal forebrain POA neurons become active at onset of sleep and release GABA at a key hypothalamic structure – tuberomammillary nucleus – that is important for increasing arousal
Brainstem system
That activates forebrain into wakefulness (a la Moruzzi and Magoun) — consists of several structures that are AChergic and NEergic
Reticular formation: Collection of cells throughout brain stem; many of which are cholinergic (ACh)
ACh neurons project to variety of structures in brain to promote wakefulness
Locus coeruleus: Major source of NE for entire forebrain; this also has stimulatory effects on alertness
Pontine system
That triggers REM sleep
Small group of cells in pons, just ventral to locus coeruleus, trigger REM sleep
Some cells project to motoneurons in the spinal cord and strongly inhibit them
This system makes muscles flaccid (i.e., not just relaxed) during REM sleep
YET, pontine neurons also project to forebrain and produce widespread activation...hence paradoxical EEG pattern during this stage of sleep
Hypothalamic system
That affects other three brain systems to determine sleep/wake
A region in the hypothalamus, including neurons that use hypocretin as a neurotransmitter, sends axons to the other three sleep centers and seems to coordinate them, enforcing the patterns of sleep.
Loss of hypocretin can lead to disorganized sleep, such as REM-like muscle atonia while still awake (in narcolepsy).
Narcolepsy: Person (or animal) has sudden, intense bouts of sleep during day – sleep lasts 5-30 minutes
Cataplexy: Sudden loss of muscle tone without loss of consciousness (probably due to aberrant activity in pontine/REM system)
In some cases can be caused by strong emotions (positive or negative)
Narcolepsy — orexin/hypocretin
Narcoleptics typically enter REM immediately upon falling asleep rather than SWS at night
Narcoleptic dogs also show immediate entry into REM sleep
In narcoleptic dogs, a mutant gene was isolated by de Lecea and Kilduff encoding for a receptor of hypocretin in 1998; at the same time Yanagisawa’s group discovered the same thing, and called it orexin
Why do we sleep?
Energy conservation
Nice adaptation
Body restoration
Memory consolidation
Energy conservation
Reduced body temperature, slower respiration, slower heart rate > reduced metabolic activity – YET, sleep reduces metabolic rate by only 5-10%
Niche adaptation
Sleep enforces adaptation to a particular ecological niche (e.g., if the animal is better at gathering food during day/night, or may avoid predators day/night, then selective pressure may have favored sleeping in the other part of day)
Body restoration
Sleep helps rebuild/restore body materials and functions
Prolonged deprivation – weakened immune system
Work at night vs. day – increased risk of cancer
Does not refer to simple wear and tear (e.g., exercise does not cause people to sleep longer)
Memory consolidation
Evidence in recent years indicates that sleep promotes memory consolidation
SWS helps in the consolidation of declarative memories (i.e., memory of factual information)
Involvement of REM sleep in consolidation of procedural memories (i.e., skill- or rule-based learning)
Treating sleep disorders
Narcolepsy and cataplexy – different medications can treat this...
Traditionally amphetamines have been used; more recently modafinil (effects of this are not well understood) is used and seems to be very effective
Sleep disorders — very young people
Two common ones in young children; night terrors and sleep enuresis (i.e., bed-wetting)
Both occur during SWS
Can be treated with drugs to decrease stage 3-4 SWS sleep or with an antidiuretic
Somnambulism (sleepwalking): More common among children but can persist into adulthood
Also occurs during SWS; patients are NOT acting out a dream
Sudden infant death syndrome (SIDS)
May be due to abnormalities in brainstem circuits that regulate respiration; especially those involving serotonin
Solution? Leave infant sleeping on back, not stomach
REM behavior disorder
Patients appear to be acting out a dream, of somewhat organized-seeming behavior
Begins around age 50 and more common among men
Onset often followed by Parkinsons or dementia
Insomnia
2 categories
1. Sleep-onset insomnia: Often caused by situational factors (shift work, time zone shifts, etc.)
2. Sleep-maintenance insomnia: More due to drugs, psychiatric, neurological problems
Sleep apnea
Respiration becomes unreliable and person may stop breathing for a minute – common cause of insomnia
Sleep apnea may lead to a variety of cardiovascular problems and even neuron death
Continuous positive airwave pressure (CPAP) machine solves sleep apnea
4 dimensions of emotion
1. Physiological: Autonomic, endocrine responses
2. Actions: E.g., laughing; attacking/fleeing in response to threat
3. Motivation: Approach or avoidance behaviors
4. Feelings: Subjective experience
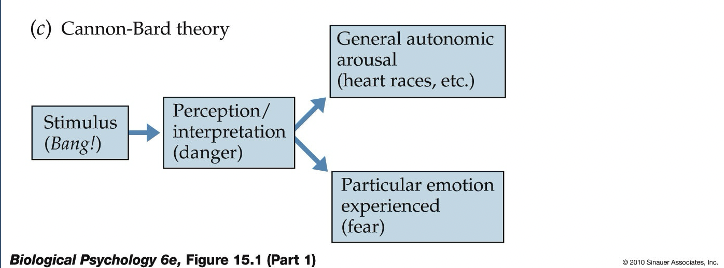
Walter Cannon
First to observe that emotional excitement was associated with the following changes:
a. Increase of adrenaline (epinephrine) in bloodstream
b. Redirection of blood flow from the viscera (internal organs) to skeletal muscles and brain
c. Increased blood pressure and glucose levels
d. Less movement of the muscles in the digestive organs
Cannon coined the term “fight-or-flight" or sympathetic response
Other investigators verified that the posterior hypothalamus played an important role in the expression of emotions
Sham rage
Resulted in a 5-fold increase in blood glucose levels and increased secretions of the adrenal gland (i.e., release of adrenaline)
Cannon showed that stimulation of the diencephalon could produce vocalizations, changes in respiration, circulation, piloerection (hair standing up on back), and coordinated movements in cats
The general idea that emerged from this work is that the diencephalon produces emotional responses but that it is normally inhibited by the cortex
Fear conditioning requires the amygdala
Sensory information related to the threat is then conveyed to the lateral nucleus of the amygdala
Information is processed through the amygdala, and then to the central amygdala for output to structures controlling different aspects of fear responses:
a. Defensive behaviors (periaqueductal gray, PAG; “central gray” on diagram)
b. Autonomic activation (through lat. hyp.)
c. Stress hormone responses (through the anteroventral bed nucleus of the stria terminalis and paraventricular hypothalamus; avBST and PVH)
Brain system of “approach” behaviors
The common feature to all these manipulation is that the ascending pathway, the medial forebrain bundle, is activated
This pathway contains dopamine neurons, arising from the ventral tegmental area (VTA) that innervate the nucleus accumbens (recall mesolimbic system)
Pleasurable experiences, or emotions pertaining to joy, happiness, all involve activation of the dopamine-nucleus accumbens pathway
VTA-DA neurons
Encode reward prediction error
More recently, evidence suggests that VTA-DA neurons play a role in predicting reward- and aversive-related cues
VTA-DA neurons show reward prediction error – they fire APs more when a reward is larger than expected, and decreased activity when no or less than predicted reward occurs
Subjective experiences of emotion
Emotions tend not to be localized in distinct parts of the cortex
A single type of emotion increases activity in various parts of the brain – but not in one single region
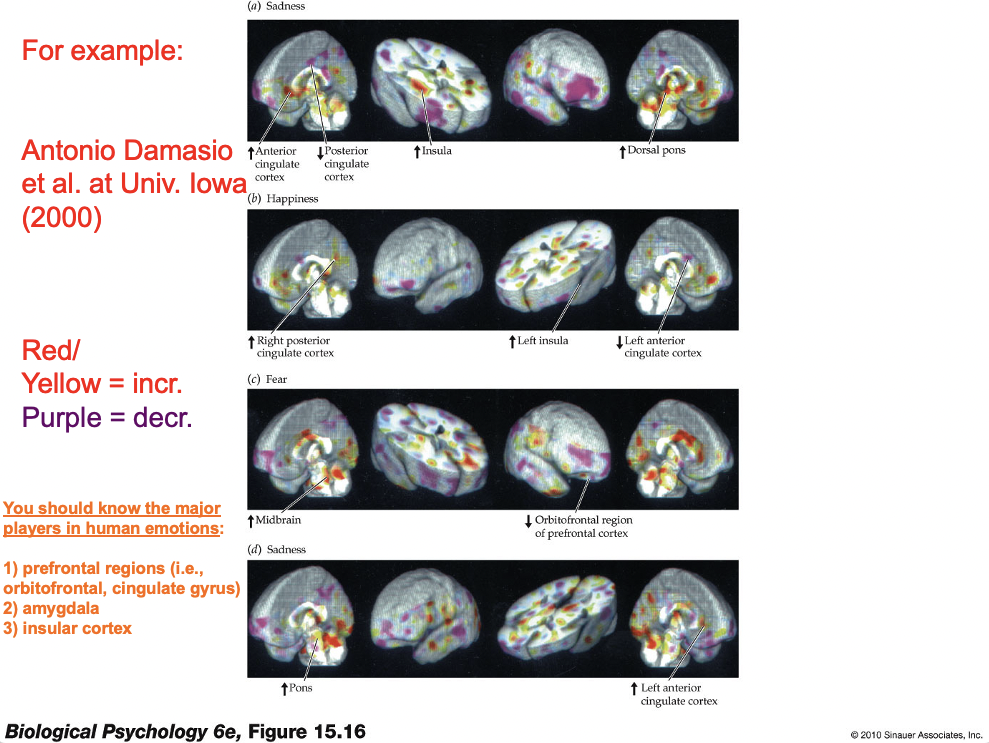
Insular cortex
Strongly activated during exposure to stimuli perceived as “disgusting” -- this part of cortex is also responsible for processing taste information (analogous to A1, V1, and S1) so this may tie in to Darwin’s ideas about emotion
Stress
Hans Selye (1907-1982): The first to popularize the use of this term
Selye (1956): Defined stress as “the rate of all wear and tear caused by life”; negative emotions were hypothesized to be one source of “wear and tear”
Selye proposed that the connection between stress and disease is highlighted by the general adaptation syndrome
Selye’s idea of stress
General adaptation syndrome has 3 phases:
1. Alarm reaction: Initial response to stress
2. Adaptation stage: Includes activation of appropriate response systems and re-establishment of homeostatic balance
3. Exhaustion stage: Occurs when stress is prolonged or severe; characterized by increases susceptibility to disease
Two important factors for stress appraisal
1. Predictability: Do you know it is coming
2. Controllability: Can you change the outcome
Both of these can reduce stress
Negative effects of chronic stress
Short Term Adaptation | Long Term Pathology |
Mobilization of energy reserves | Myopathy, fatigue, type 2 diabetes |
Increased cardiovascular output | Hypertension |
Suppression of digestions | Ulcers, irritable bowel disorder |
Suppression of growth | Psychosocial dwarfism |
Suppression of reproduction | Amennorrhea, impotency, loss of libido |
Altered immune function | Immunosuppression, risk of infection |
Heightened awareness, cognition | Synaptic pruning (i.e., cortex, hippocampus) |
ALL ARE MEDIATED BY INCREASED CORTISOL
Psychological dwarfism
Growth failure that results from psychological and social factors is mediated through the CNS and its control over hormone system (Green et al., 1984)
When such children are removed from stressful circumstances, many begin to grow rapidly (asterisks)
Prolonged cortisol secretion from HPA hyperactivity may inhibit growth hormone (GH) release, which is necessary for physical development and growth in children
Effects of early life stress
More recent research showed that these early maternal experiences produce epigenetic changes that mediate alterations in stress reactivity during adulthood
Early life trauma in humans is also associated with a greater risk of major depressive disorder later in life – and is now thought to have an epigenetic basis
Prolonged elevations in CORT in rats cause atrophy in pyramidal neurons and destroy excitatory synapses in the hippocampus and prefrontal cortex
Cushings disease
Endocrine syndrome where cortisol levels are chronically elevated; these patients show massive hippocampal shrinkage and cell loss
(Prefrontal cortex has never been examined in Cushings disease patient, but probably this too is affected)
Post-traumatic stress disorder (PTSD)
Occurs in some people after certain crises or terrifying experiences
Symptoms: a) frequent distressing recollections, b) nightmares, c) avoidance of reminders of the event, d) exaggerated arousal in response to noises and other stimuli
Stress-related mental illnesses
Main categories: a) major depressive illness, b) reactive depression, c) anxiety disorders, d) post-traumatic stress disorder (PTSD)
There is a substantial degree of comorbidity (i.e., overlap) between the occurrence of these disorders
Mental illness — historical origins
1930s: Experiments on frontal lobe lesions performed in chimps inspired Egas Moniz to attempt similar operations in humans
Moniz was intruiged by the calming influence that such lesions were reported to produce
Thus began the era of psychosurgery – the use of surgical manipulation to treat severe mental illness
1970s-present: Occasional examples where surgeries (usually discrete lesions) were performed in attempts to relieve certain mental illnesses; these have had relatively limited value
Although epilepsy is not a mental illness, focal lesions have proven more successful in alleviating frequently occurring seizures
Lobotomy
Frontal lobe lesions
During its heyday, 10-50K patients in US were estimated to have had this surgery
Surgery was supposed to induce relaxation and calm individuals with severe or intractable mental disorders
Side effects were not so good – among many, mood swings, change in personality
Initially, there was much enthusiasm for this procedure, although as side effects became more noteworthy and controlled assessment were made, the procedure was eradicated
Walter Freeman
American psychiatrist that performed and aggressively advocated the prefrontal lobotomy; he probably deserves much of the blame for its overuse
Lobotomies are no longer done, but other lesion-life procedures are still used in rare cases
Characteristics of schizophrenia — positive symptoms
Loose association, tangential thinking
Trouble with making abstractions – no intuition on how to get to the right level of abstraction; usually too concrete in thinking
Delusions – thinking things that can not be true, e.g., as being involved in historical events or interacting with famous people
Paranoia
Structured hallucinations – usually auditory; these have a well-defined structure and content, such as voices
Characteristics of schizophrenia — negative symptoms
Social withdrawal: Abnormal social associations
Absence of affect: Dampened emotionality
Violent tendencies? Not really, but if anything, SZ sometimes involves self-injurious behaviors; upward of half of SZ patient will attempt suicide at some point
Biological/brain differences in SZ — genes
Highly heritable, but hard to link with mechanism – variations genes for MHC, DISC1, and COMT genes have been identified
Biological/brain differences in SZ — brain abnormalities
Live imaging – decreased frontal cortex activity during memory tasks, ventricular enlargement; some postmortem studies show synapse loss in frontal cortex
Biological/brain differences in SZ — developmental
As noted – may aggravate symptoms, but not ultimate cause (e.g., viral infection, poor nutrition of mother during pregnancy, nutritional premature birth, low birth weight, complications during delivery, adolescent marijuana use)