cpp mt
1/112
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
113 Terms
three parts of the pharmacist patient care process (PCPP)
collaborate, communicate, document
five step process of pharmacist patient care process
1. collect
2. assess
3. plan
4. implement
5. follow-up: monitor and evaluate
SOAP
subjective, objective, assessment, plan
three part of assessment
first: state problem identified
second: list findings that support your conclusion
third: state short and long term goals
examples of short and long term goals
short: eliminate symptoms of UTI
long: cure infection
monitoring outcomes is part of
plan
checklist to ensure SOAP is being used efficiently
-all subjective and objective info is pertinent
-med-related problem list is prioritized
-assessment is clear and concise, explains thought process, answers 'why' and includes goals
-plan includes specific recommendations and monitoring parameters
who is involved in community pharmacy services
ancillary help --> technicians --> interns --> pharmacists
ancillary help
maintain stock, and medical records, type labels, count and prepare dosage units, hand deliver completed prescriptions to patients
unlicensed personnel in community settings are not allowed to
Receive verbal prescription orders Interpret or evaluate a prescription Measure, weigh, compound or mix ingredients Counsel patients Make clinical recommendations
pharmacy interns
can perform all the duties of a pharmacist but must be supervised by a pharmacist at all times
HIPPA
Health Insurance Portability and Accountability Act
pharmacists/pharmacy is a covered entity
anyone who provides treatment, payment and operations in healthcare
covered entity
A health plan, a healthcare clearinghouse, or a healthcare provider who transmits any health information in electronic form in connection with a transaction
collect: patient profile
first and last name, address, DOB, gender, phone number/email, allergies, chronic diseases/conditions, medications, other relevant counseling information
patient profiles must be maintained for
5 years from the most recent entry
collect: insurance information
• Bank identification number: BIN #• Processor control number :PCN #• Identification number• Group codes• Medical vs. Pharmacy coverage
additional information to collect
-safety cap
-delivery services
-contact preferences
Any pharmacy in a group larger than 8 (NYS) or larger than 4 (NYC), is required
Free written and oral "translation" services
ways to receive and prescription
in person (paper), verbal, fax, electronically
required documentation for verbal prescrptions
-the date
-initals of who is receiving the call
-full signature of controlled safety
Controlled Substances Act (CSA)
Legislation in the US that defines illegal drugs and classifies them by Schedules
five schedules based on
accepted medical use
abuse potential
likelihood of causing dependence when abused
CI vs CV
CI: basically illegal drugs
-no acceptable medical use
-high potential of abuse
-lack of accepted safety for use under medical supervision
CV: low potential for abuse
limited quantities of narcotics
controlled substances: law
Must be filled within 30 days of date ordered
Cannot be filled for more than a 30-day supply
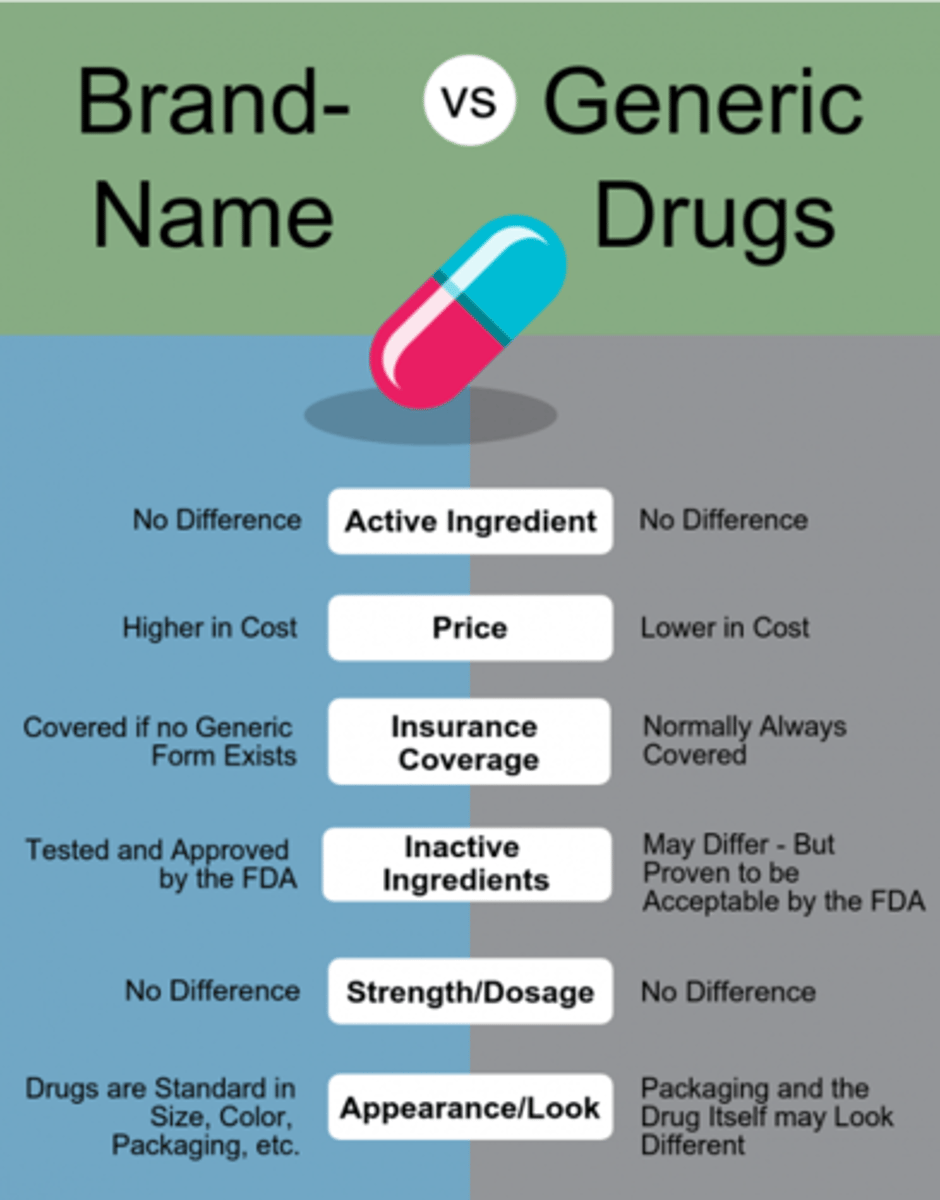
brand vs generic
generic: Same as a brand name drug in dosage, safety, strength, how it is taken, quality, performance, and intended use
contain same amount of API
two parts of preventing medication errors
technical process and clinical process
three technical ways to minimize medication errors
• "Tall Letters": mix of lower and uppercase letters.• Verify National Drug Codes (NDC#) against labels and bottles• Utilize Bar Codes
prescription label standards
1. patient name
2. drug name
3. drug strength
4. directions
use biggest font
full requirements of a label
-name and address of patient
-name and address of the owner of the pharmacy in which it was dispensed
-date compounded
-Rx number
-name of prescriber
-directions
-if generic, name of manufacturer
-name and strength of drug
these are not required by law to be on a label
refills and quantity
orange label
CONTROLLED SUBSTANCE, DANGEROUS UNLESS USED AS DIRECTED
auxiliary labels
Provide additional information, such as special instructions, warnings, or storage conditions to the patient
Drug Utilization Review (DUR)
A system of drug use review that can detect potential adverse drug interactions, drug-pregnancy conflicts, therapeutic duplication, drug-age conflicts, and so on
Internet System for Tracking Over-Prescribing -Prescription Monitoring Program (I-STOP - PMP)
required to submit controlled substance dispensing data to bureau of narcotic enforcement
FDA mandated medication guides
Addresses issues specific to a particular drugsand/or drug class
Omnibus Budget Reconciliation Act (OBRA)
A pharmacist or pharmacy intern must provide patient education (counseling) before -Before dispensing a medication to a new patient of thepharmacy
-Before filling a new prescription for an existing patient ofthe pharmacy
-if there are new dose, strength, route, or directions
An offer to provide counseling must be made every time a patient
has a prescription refilled or has a prescription filled for a medication therapy that has been reauthorized by a prescriber
daily record
-Reports all new and refilled prescriptions dispensed
-signed by pharmacist whose initials appear on prescriptions
-must be kept for 5 years
evidence-based medicine
medical care based on the latest and most accurate clinical research
examples of primary sources
• Clinical research studies• Scientific experiments• Journal articles
examples of secondary sources
Synthesize findings from multiple primary resources: searchable databases
• Embase• PubMed (free)
tertiary sources
Include summarized information from both primary and secondary sources, typically condensed into a more digestible format
tertiary sources examples
• Textbooks• Compendia (Lexicomp, Micromedex)• Package inserts• Websites (CDC, Clinicaltrials.gov)• Other online databases (UpToDate)
Prescribing Information (PI) or United States Prescribing Information (USPI)
includes FDA approved indications, side effects, adverse reactions, contraindications, and other important information
Recommend reliable, high-quality health information geared for theaverage consumer, such as
• Health care organizations (Mayo Clinic, Cleveland Clinic)
• Disease or professional societies (American Diabetes Association)
• Consumer-specific sections from tertiary resources (Micromedex, Lexicomp)
Hundreds of drug interactions have been documented; few are clinically significant enough to be
contraindicated or require a change in dosage
pharmacokinetics
the effect the body has on the drug as it travels through the absorption, distribution, metabolism, and excretion (ADME) processes
Pharmacokinetic interactions occur when one drug alters the
ADME processes of another drug
Absorption from the gastrointestinal (GI) tract may be influenced by:
• Agents that bind drugs• Agents that increase/ decrease GI motility• Change in gastric pH• Drugs that alter the p-glycoprotein and organicanion transporters in the intestine
Distribution can be altered by drugs that
compete for binding sites on plasma proteins
Agents can change the size of the physical compartment in which
another drug distributes
Highly protein-bound drugs can be displaced from binding sites on albumin,leading to
increased drug concentration in the blood
excretion
removal of a drug from the body
Excretion of drugs by the kidney can be changed by drugs that
reduce renal blood flow or inhibit specific renal transport mechanisms
Drugs that alter urinary pH can alter the ionization state of drugs that are weak acids or weak bases, leading to
changes in renal tubular reabsorption
metabolism
the process of converting a drug into a form that can be excreted
most pharmacokinetic drug interactions occur during
metabolism
metabolism: Enzyme-catalyzed transformation reactions occur in
intestine and liver
The metabolism of drugs may beaffected by other agents thatinfluence the hepatic drug-metabolizing enzymes, especially
cytochrome P450 isoenzymes
More than 50 CYP enzymes havebeen identified and the most clinically important are
CYP2C9, CYP2C19, CYP2D6, and CYP3A4
enzyme inducers
decrease the concentration of substrate drugs --> more enzymes
enzyme inhibitors
increase the concentration of the substrate drugs --> cause less functional enzymes and/or decrease in rate of drug metabolism
pharmacodynamics
the effect or change the drug has on some type of organism (such as the human body)
pharmacokinetics vs pharmacodynamics
Pharmacokinetics: What the BODY does on the drug
(movement of drug through body)
Pharmacodynamics: What the DRUG does on the body
pharmacodynamic interactions
additive, synergistic, antagonistic, potentiation
antagonism
interactions based on opposing actions or effects
additive effects
the algebraic summing of the effects of two drugs
synergism
when the result of the interaction is greater than the sum of the drugs used alone
potentiation
When one drug's effect is increased by another agent that has no such effect
pharmacotherapy
Area of pharmacy practice that ensures the safe, appropriate, and economical use of medications
Newly approved medications should be used only if
there are clear advantages over older medications
A medication should not begiven by injection (IV, IM, SQ) when
giving it by mouth would be just as effective and safe.
avoid the "prescribing cascade"
treating side effects as a new disease
Before medications are used, these interventions should be made
lifestyle modifications or non-pharmacologic interventions
three primary components of a drug therapy problem
Undesirable event or risk of an eventexperienced by the patient
The drug therapy associated with theproblem
The relationship that exists between the undesirable patient event and the drug therapy
contraindicated
should not be used because it may be harmful to the person
Joint Commission
an independent, not-for-profit organization that evaluates and accredits healthcare organizations
-maintains standards of healthcare
-"checks-up" on facility
-publishes yearly patient safety goals
centralized vs decentralized pharmacies
CareSatellitePediatricSatelliteCentral Centralized One pharmacy that serves the entirehospital Decentralized One central pharmacy and Many "satellite" pharmacies Located near patient units
pharmacy and therapeutics committee (in hospital)
-chaired by a physician
-make important decisions/changes for the hospital formularies, policiesand procedures
role of pharmacist on medical team
-order, writing, medication reconciliation
-collab on medication therapy managment
-hospital initiatives
medication reconciliation
Process of identifying the most accurate list of all medications apatient is taking Includes name, dosage, frequency, and route
medication reconciliation involves comparing the patient's current list of medications against
the physician's admission, transfer, and/or discharge orders
responsible for as many as 50% of all medication errors and up to20% of adverse drug events in the hospital
poor communication of medical information
steps in the medication order process
ordering (clinician) --> transcribing (not necessary for electronic) --> verifying and dispensing --> administration (nurse)
CPOE
computerized physician order entry
the order is entered into
patient profile
when there is a problem with orders, the
prescriber must be contacted
though administration of meds is a nursing responsibility, it becomes important to the pharmacist when
we are monitoring levels of specific medications
medication error
Any preventable adverse drug event involving inappropriate medication use by a patient or health care professional; it may or may not cause the patient harm.
prohibited abbreviations: U
unit
prohibited abbreviations: IU
international unit
prohibited abbreviations: QD and QOD
daily and every other day
prohibited abbreviations: .X and X.0 mg
0.X mg and X mg
prohibited abbreviations: MS, MS04 and MgS04
morphine sulfate and magnesium sulfate
medical record
-Chart/file created for newly admitted hospital (inpatient) or clinic (outpatient/ambulatory) patients
-Serves as a legal document that documents the course of treatment
disadvantages of electronic medical records (EMR)
Cost of system implementationTransitions in care- from different health systemsTrainingPower outage/technical issuesSecurity/risk for data breach“Cut and paste” errors
RHIO - regional health information organization
Group of organizations that share healthcare-related information Allow healthcare provides greater patient access to information generated atother facilities
healthix
Largest public HIE (health info exchnage) serving NYC and Long Island
retention of Medical Records in NY forHospitals
Must be retained in their original or legally reproduced form for: A period of 6 years from the date of discharge Obstetrical records and children's records for at least 6 years or until thechild is age 21, whichever is longer At least 6 years after death