SL Chem - Unit #2: Combustion and Hydrate Analysis
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
Combustion Analysis
A method used to determine an elements % composition
Combustion analysis process…
Hydrocarbons are burned in pure oxygen to produce carbon dioxide and water
CuO oxidises C and CO which forms CO2
All the hydrogens form H2O
The mass of H2O and CO2 are absorbed separately and their masses are than used to determine their percentage composition
This helps determine the amount of C and H in the fuel that was being used to burn
Hydrate Analysis
Hydrates are ionic compounds with water incorporated into their crystal structure
NOT LIKE AN AQUEOUS SOLUTION
The analysis is done by heating the hydrate until it’s an anhydrous
Percentage of Water in the hydrate
Normal percent composition formula but for the whole water molecules multiplied by its coefficient
Mass of water in a specific weight of the compound
Using the percentage of water divide by 100, then Multiply by the weight of the compound
Formula
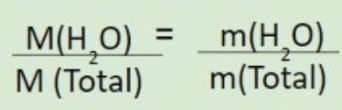
Coefficient on the Hydrate
Write down the balanced equation
Write down the mass of the hydrate and the ionic compound that forms after being heated
Determine the mass of the water that comes off after being heated using the law of conservation of mass
Solves for the moles of the compound and water
Divide the both moles by the lowest mole ratio
The ratio that you get is then the coefficient on water