6.1.3 Carboxylic Acids and Esters
1/116
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
117 Terms
How are carboxylic acids formed from primary alcohols?
complete oxidation of primary alcohols: heat under reflux with acidified dichromate
What is the carboxylic acid functional group?
COOH
What colour change acompanies the formation of carboxylic acids from primary acohols?
orange to green
Which carboxylic acids are soluble in water?
the first 4
Describe the polarity of carboxylic acids?
the carboxyl group is polar
the hydrocarbon chain is non-polar
Which part of the carboxykic acid attracts water?
carboxyl group (COOH)
Draw a diagram of the carboxyl group with water?
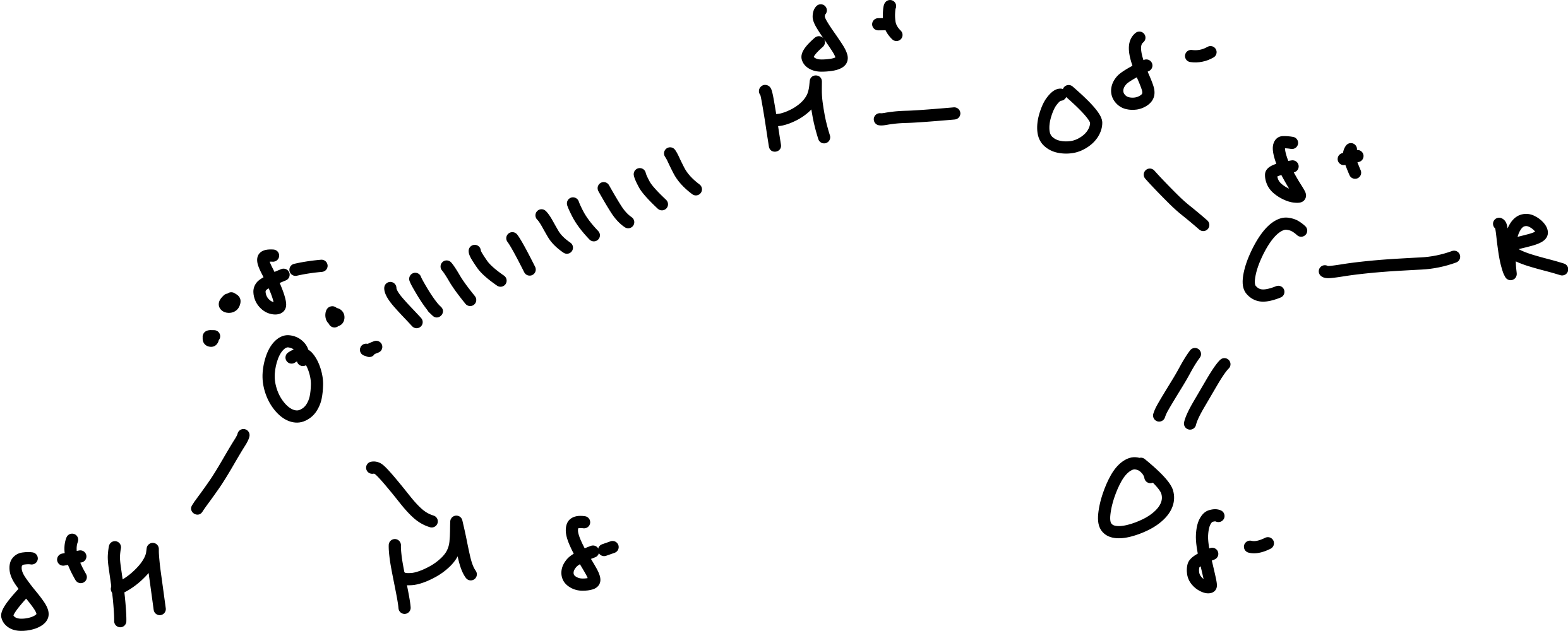
How does solubility of the carboxylic acid change as the length of the hydrocarbpon chain increases?
as it increases, solubility decreases
Why does the solubility of carboxylicc acids decrease as chain length increases?
a greater proportion of the molecule is hydrophobic
Define acid
proton donor
Define strong acid
acid that completely dissociates into its ions
Define weak acid
an acid that partially dissociates into its ions in aqueous solutions
What type of acids are carboxylic acids?
weak acids
Write the equation for the dissociation of ethanoic acid
CH3COOH ⇌ CH3COO- + H+
Describe the equilibrium position of ethanoic acid
far left
Why is the equilibrium position of ethanoic acid dissociation far to the left
the concentration of protons is low compared to the concentration of undissociated acid
How do you calculate acid dissociation constant?
[H+][A-] / [HA]
What does acid dissociation constant indicate?
extent of acid dissociation
When will Ka be a large number?
when [H+] and [A-] are large (strong acid)
What does a Ka vaue greater than 1 suggest?
equilibrium is to the right
When will Ka be a low value?
when [H+] and [A-] are low
What does a Ka value of less than 1 suggest?
equilibrium lies far to the left
How does Ka change as acids get stronger?
it gets larger
Draw the equation for propanoic acid and sodium oxide

Write the equation for propanoic acid and sodium oxide
propanoic acid + sodium oxide → odium propanoate + water
What is the observation when propanoic acid and sodium oxide react?
solid dissolves
Draw the equation for propanoic acid and sodium hydroxide

Write the equation for propanoic acid and sodium hydroxide
propanoic acid + sodium hydroxide → sodium propanoate + water
What is the observation when propanoic acid reacts with sodium hydroxide?
no visible change
Draw the equation for propanoic acid and magnesium carbonate

Write the equation for propanoic acid and magnesium carbonate
propanoic acid + magnesium carbonate → magnesium propanoate + water + carbon dioxide
What is observed when magnesium carbonate reacts with propanoic acid?
solid dissolves and fizzing
What is the chemical test for the presence of a carboxylic acid?
sodium carbonate: positive result shows fizzing and the solid dissolves
Draw the equation for propanoic acid and calcium
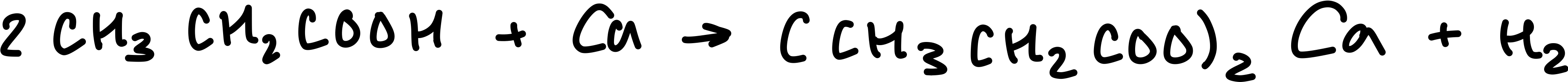
Write the equation for propanoic acid and calcium
propanoic acid + calcium → calcium propanoate + hydrogen
What is observed when calcium reacts with propanoic acid
solid dissolves and fizzing
What type of reaction is that between a carboxylic acid and a metal?
redox
Draw calcium propanoate
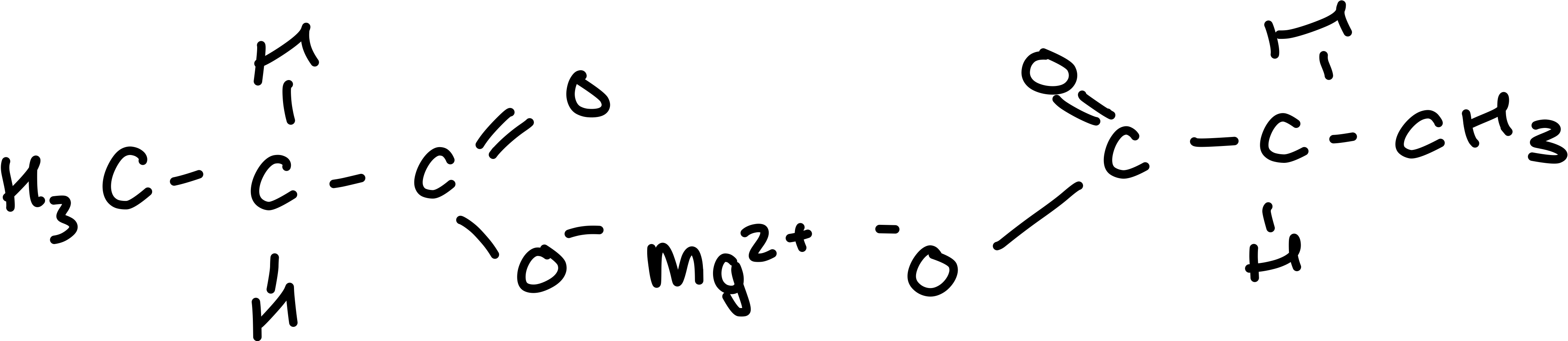
Draw the equation for propanoic acid and ammonia

Write the equation for propanoic acid and ammonia
propanoic acid + ammonia → ammonium propanoate
What would be observed when ammonia reacts with propanoic acid
no visible change
Define salt
the compound produced when the H+ of an acid is replaced by a positive metal or ammoium ion
Define base
a proton acceptor
Define alkali
base that dissolves in water and releases hydroxide ions in aqueous solutions
What is the ester functional group?
-COO
Draw the ester functional group?
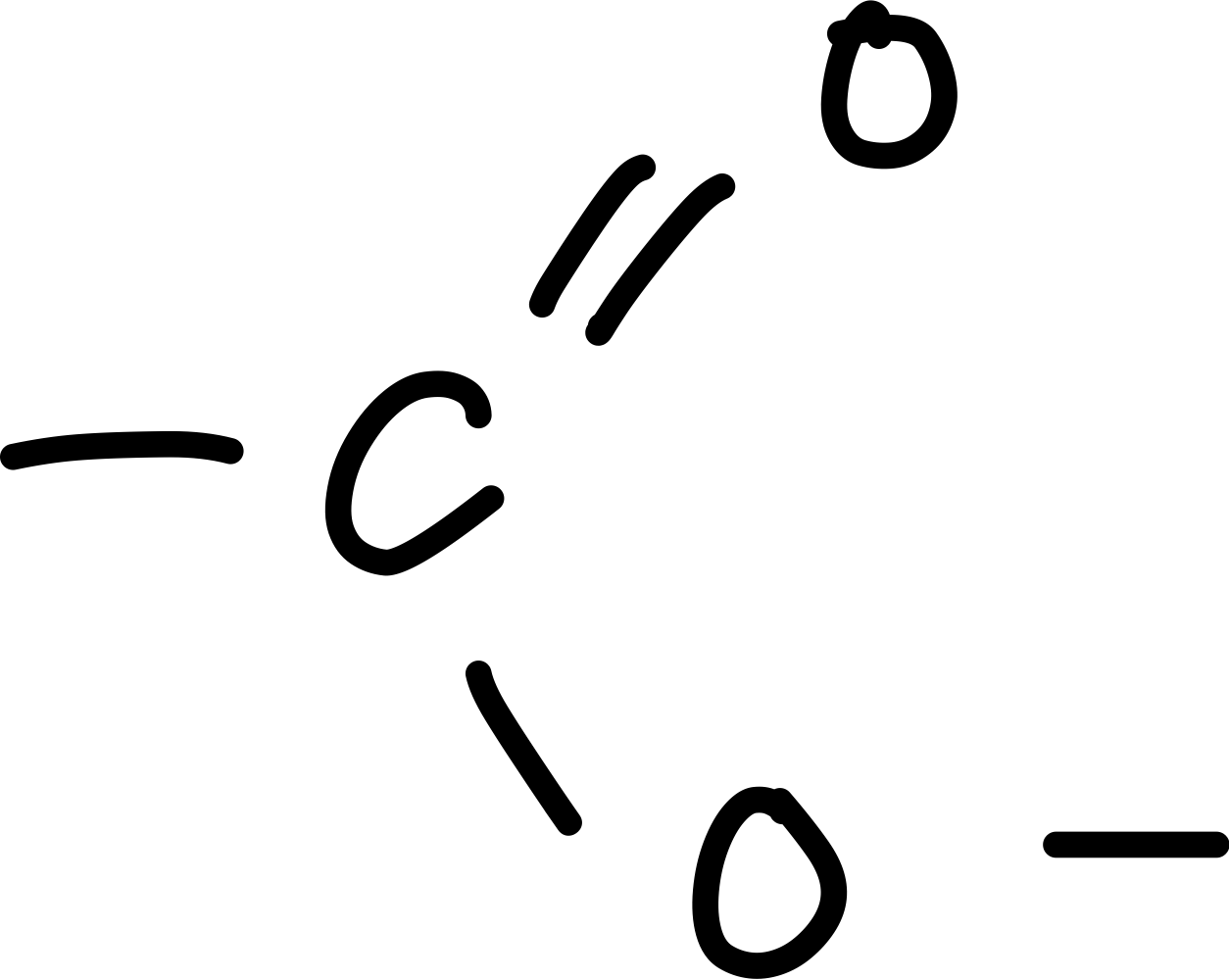
What are the reagents for the preparation of an ester fro a carboxylic acid and an alcohol?
carboxylic acid
alcohol
What are the conditions needed for a carboxylic acid and alcohol to react?
concentrated H2SO4
heat
What catalyst is used to react a carboxylic acid and alcohol?
concentrated sulfuric acid
What is the reaction between a carboxylic acid and alcohol called?
esterification
What type of reaction is esterification?
condensation
Why is esterification a condensation reaction?
a water molecule is lost when the carboxylic acid and alcohol molecules join together to form an ester
What is lost when a carboxylic acid and alcohol react to form an acid?
a water molecule
Is esterification reversible or irreversible?
reversible
Describe the yield of ester when formed from a carboxylic acid and alcohol?
low
Why is the yield of ester when formed from a carboxylic acid and an alcohol low?
the reaction is reversible
How are esters named?
alkyl (from the alcohol) alkanoate (from the carboxylic acid)
What is the name of the ester formed when methanol and propanoic acid react?
methyl propanoate
Draw 2-butyl methanoate
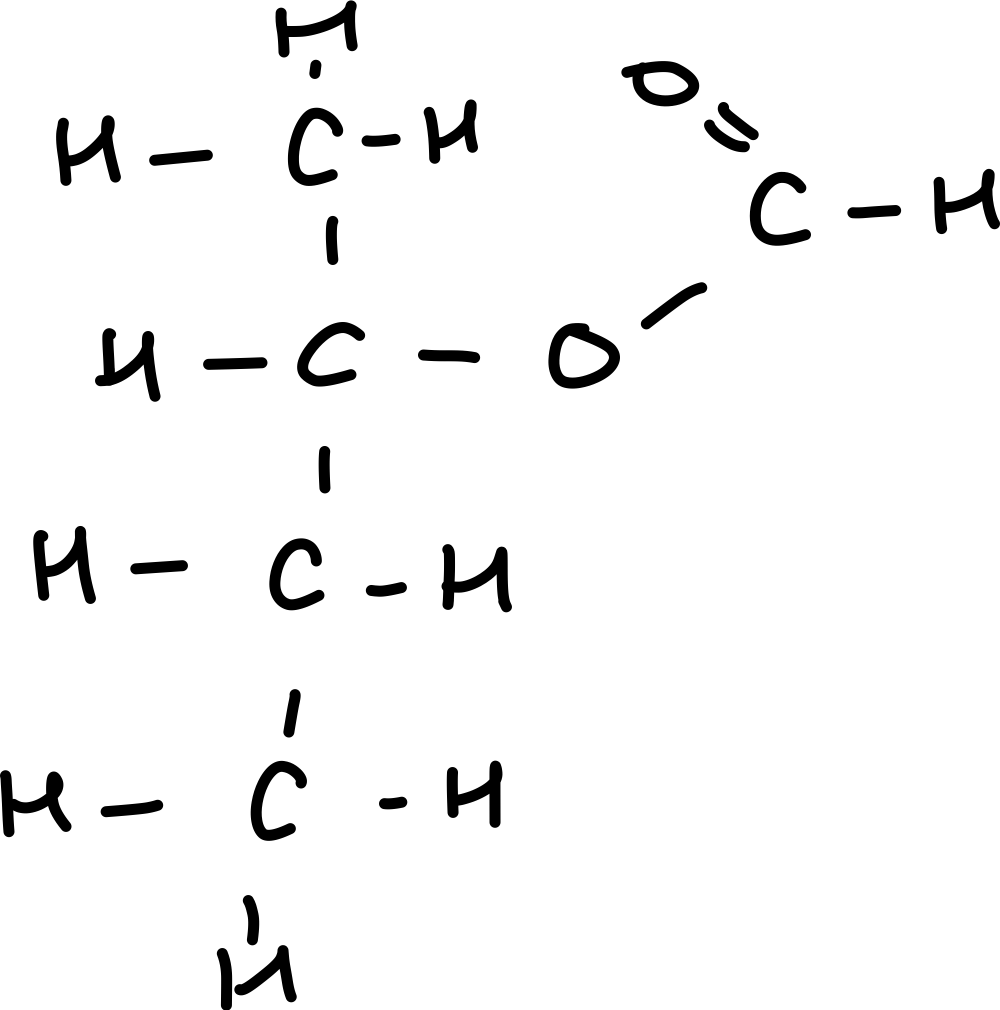
Draw ethyl-2-methyl butanoate
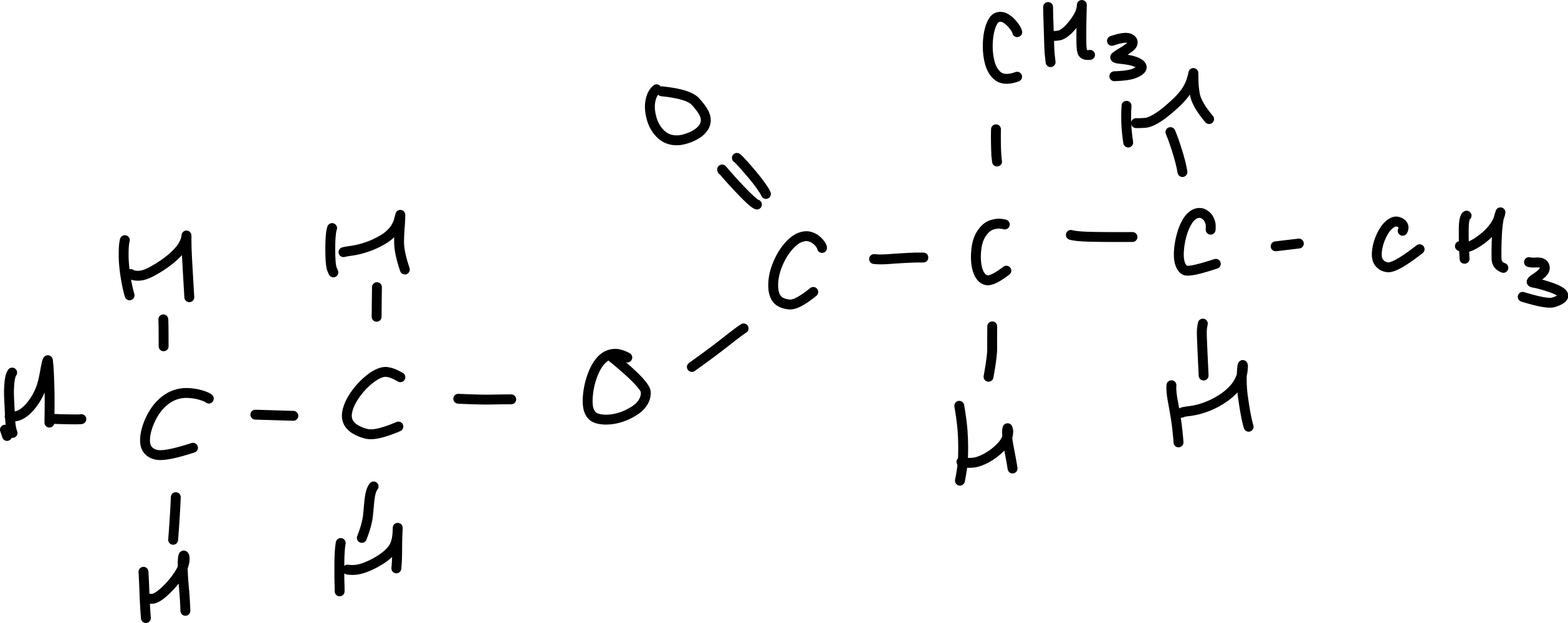
Draw 2-propyl 3-methyl heptanoate
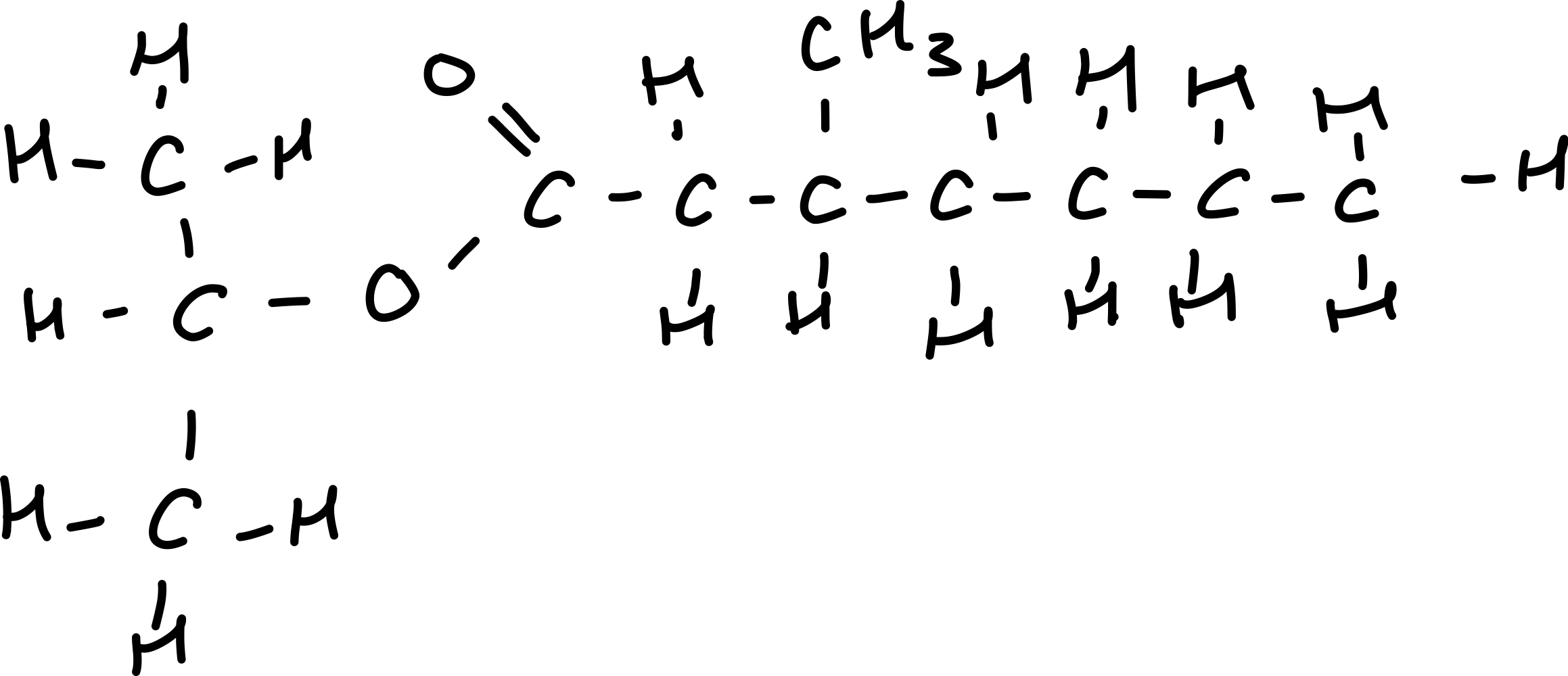
What reagents are needed for the formation of an ester from an acid anhide?
acid anhydride
alcohol
What conditions are needed for the reaction between an acid anhydride and alcohol?
gentle heat
anhydrous conditions
Draw ethanoic anhydride
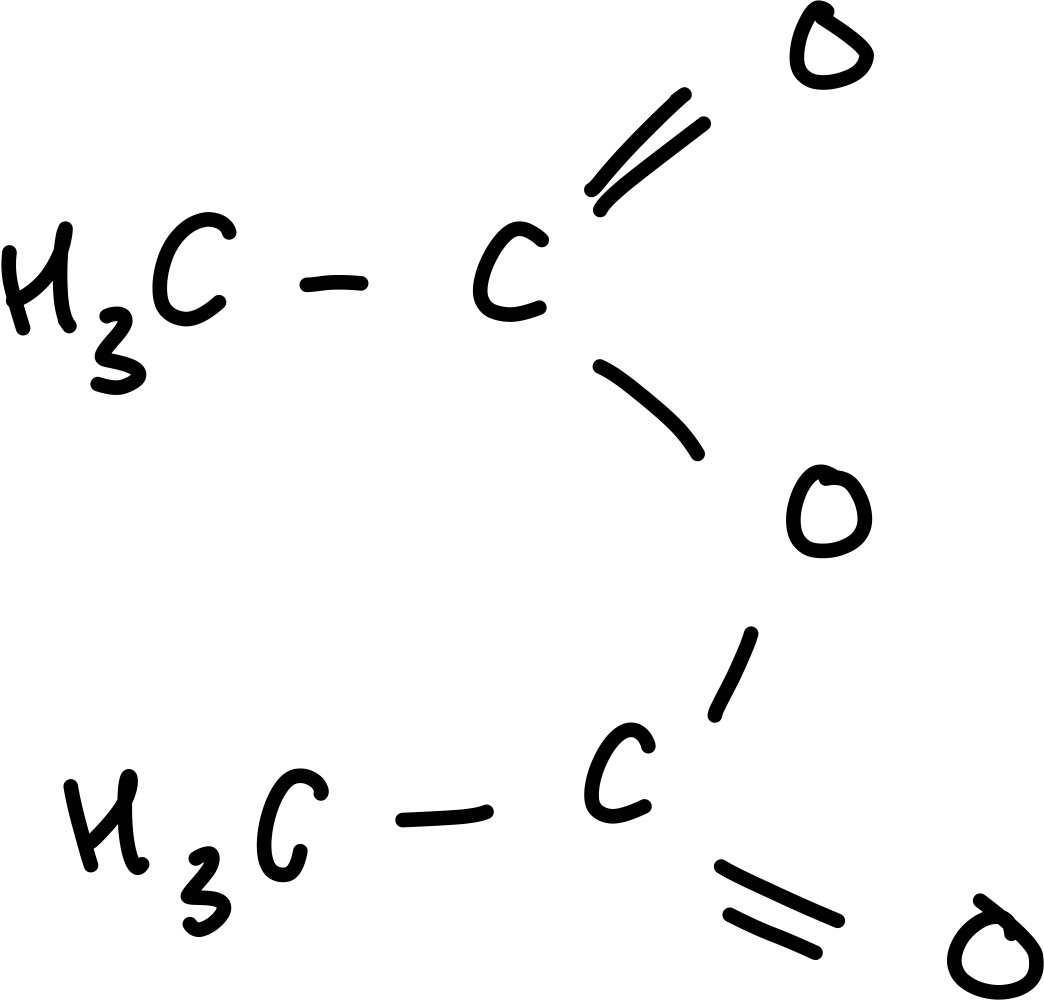
Compare the yield of ester from an acid anhydride compared to esterification
higher
Why is the yield of an ester from an acid anhydride greater?
it is irreversible
IS the reaction between an acid anhydride and alcohol reversible?
no
What are the products from the reaction of an acid anhydride and alcohol
ester
carboxylic acid
Draw the reaction between propanoic anhydride and methanol
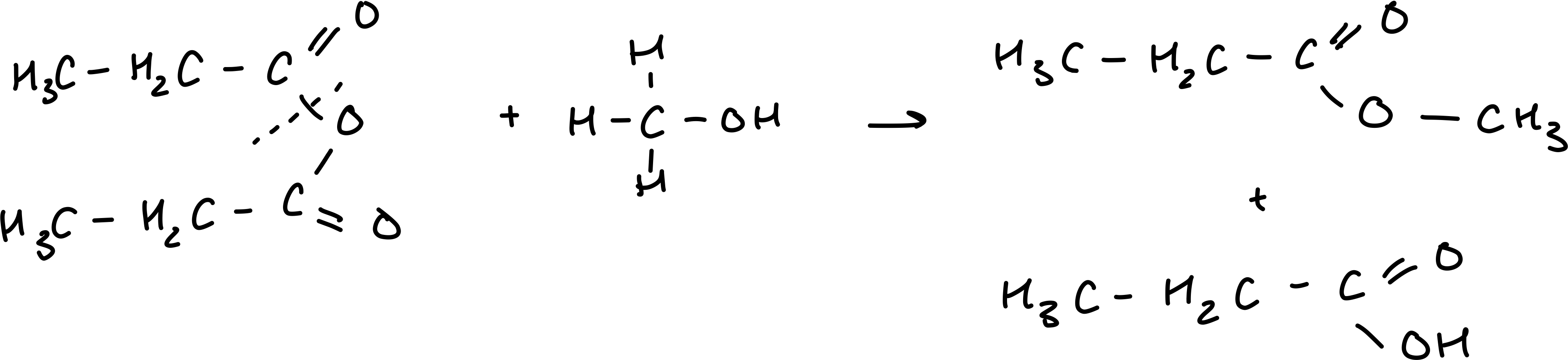
When the acid anhydride is non-symetrical, how many possible products are there in the reaction with an alcohol?
4
Draw the reaction between butanoic anhydride and pental-2-ol
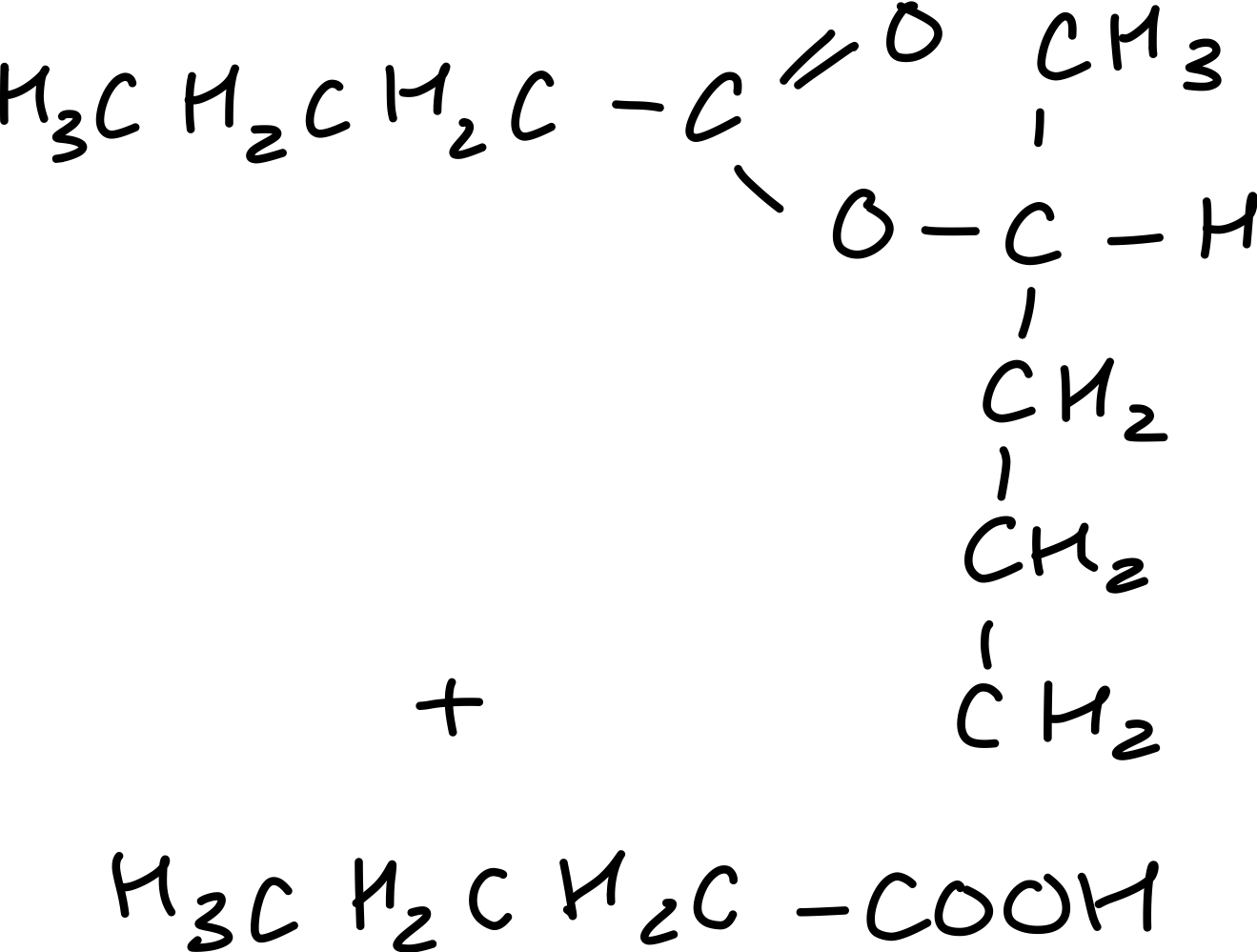
Define hydrolysis
the breaking of a covalent bond by its reaction with water
What happens when an ester is hydrolysed?
the ester group reacts with water
Which bond breaks when an ester is hydrolysed?
C-O sigma bond
Give 2 types of ester hydrolysis
acid catalysed
base catalysed
What reagents are needed for acid catalysed hydrolysis?
aqueous acid
ester
What are the conditions for acid-catalysed hydrolysis?
heat under reflux
What is formed from acid hydrolysis of an ester?
carboxylic acid
alcohol
Is acid hydrolysis of an ester reversible?
yes
What are the reagents for base catalysed hydrolysis?
aqueous alkali
ester
What are the conditions for base-catalysed hydrolysis?
heat under reflux
What are the products of base catalysed hydrolysis of an ester?
carboxylate salt
alcohol
Is base-catalysed hydrolysis of an ester reversible?
no
Why is base catalysed hydrolysis of an ester irreversible?
formation of the carboxylate salt
What is the acyl chloride functional group?
COCl
Draw the acyl chloride functional group
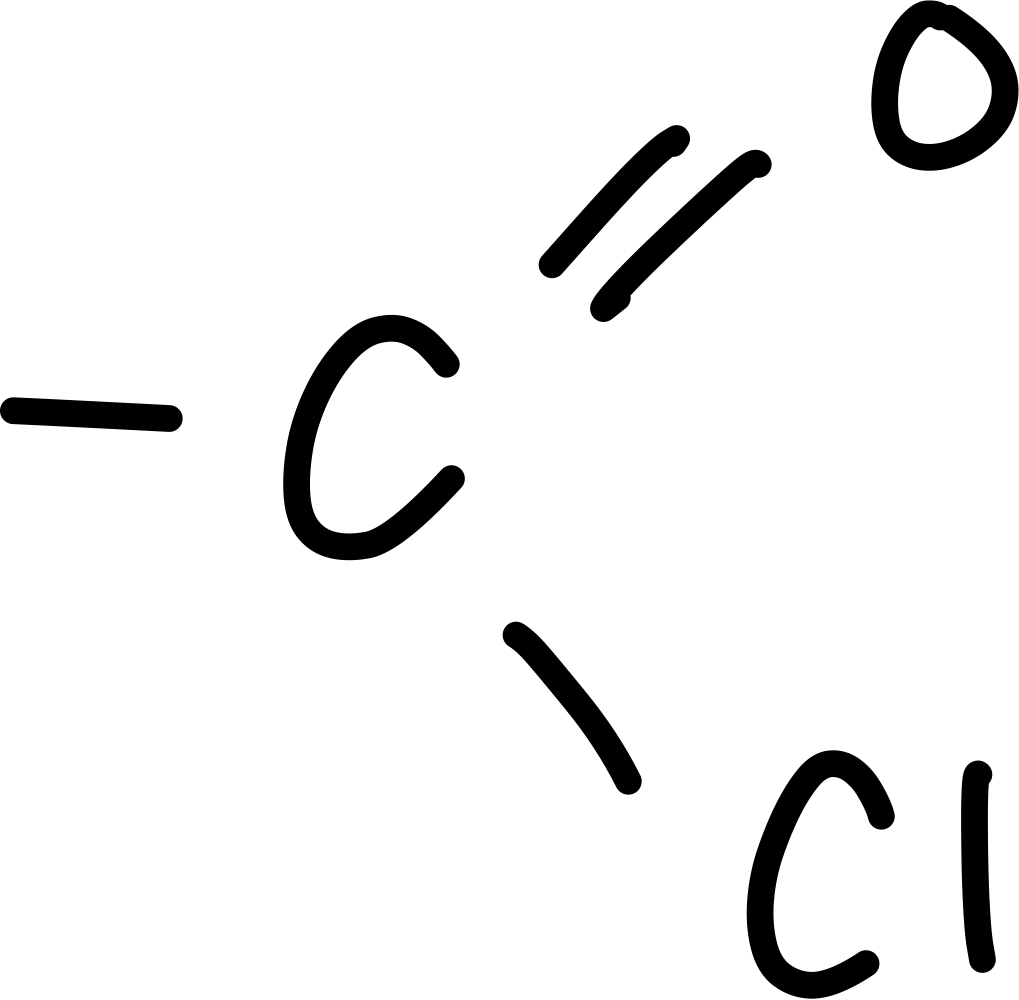
What is always carbon 1 of an acyl chloride
The carbon that is part of the acyl chloride functional group
How are acyl chlorides named?
-oyl chloride (e.g. ethanoyl chloride)
How are acyl chlorides formed?
from the reaction of a carboxylic acid and SOCl2
What reagents are needed for the formation of acyl chlorides?
carboxylic acid
SOCl2
What conditions are needed for the formation of acyl chlorides?
anhydrous conditions
What is the equation for the formation of ethanoyl chloride?
ethanoic acid + SOCl2 → ethanoyl chloride + SO2 + HCl
After the reaction between the carboxylic acid and SOCl2 how is the acyl chloride obtained?
it is warmed gently and separated by distilation
Why must the preparation of acyl chlorides happen in anhydrous condutions?
acyl chlorides react readliy with water
What are anhydrous conditions?
apparatus completely dry
ethanoic acid not aqueous
water not allowed to enter the apparatus during the experiment
How are the openings of apparatus treated to stop water from entering?
they are protected by a drying tube that contains a drying agent (CaCl2)
What will a drying tube contain?
drying agent
What is a drying agent?
an anhydrous inorganic salt that readily takes up water to become hydrated
Give 2 examples of drying agents
CaSO4
MgSO4
Why are acyl chlorides useful in synthesis?
they are very reactive and can easily be converted into many different products