Equations for Mcat
1/256
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
257 Terms
sin(90)
1
sin(60)
0.9 (√3 /2)
sin(30)
0.5
sin(45)
0.707 (√2/2)
cos90
0
sin0
0
cos60
0.5
cos30
0.9 (√3/2)
cos(45)
0.707 (√2/2)
cos(0)
1
Newtons second law
F=ma
Newtons third law
Fa on b = -Fb on a
Gravitational force between two objects
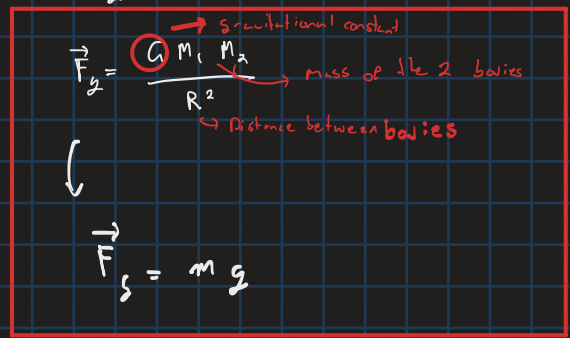
Kinetic friction

Static Friction
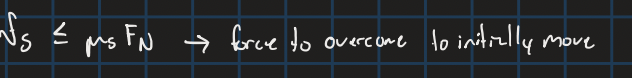
Hookes law
F = -kΔx
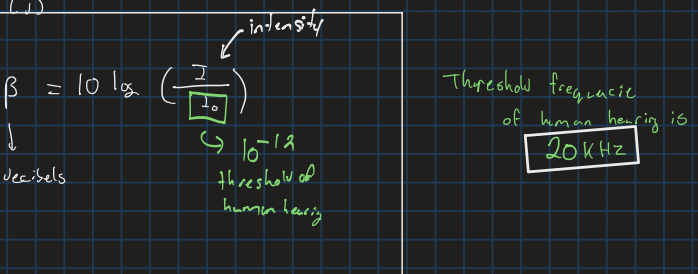
Frequency threshold for human hearing
20kHz
distance with contsnat velocity
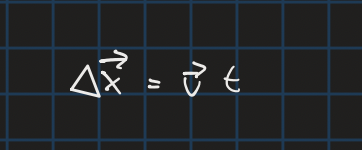
acceleration from velocities and time
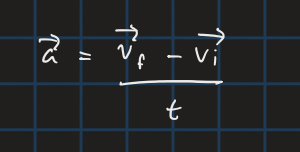
final velocity from initial, acceleration and displacement

final velocity from vi and a and t
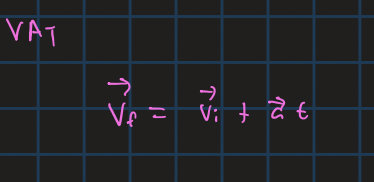
displacement from vi and a and t
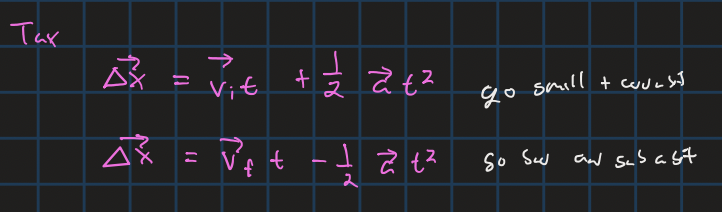
Vx in projectile motion
Vcosθ
Vy in projectile motion
Vsinθ
acceleration inclined plane
gsinθ
normal force inclined plane
mgcosθ
gravity down plane
mgsinθ
frcition inclined plane
μmgcosθ
Energy of a wave
and h = 6.626×10-34

velocity of a wave
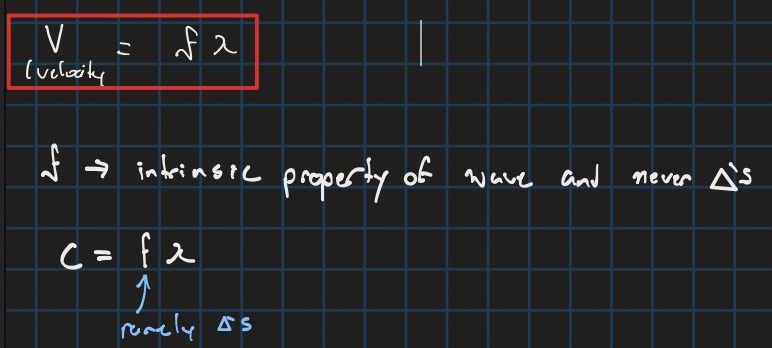
Rydberg equation
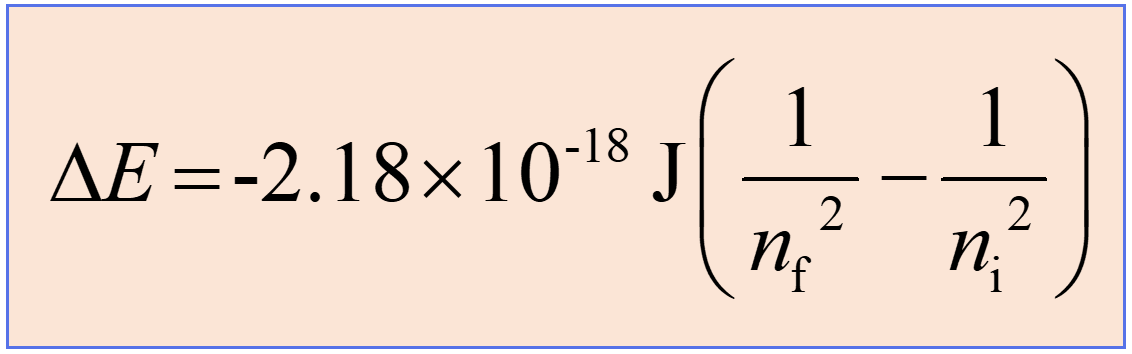
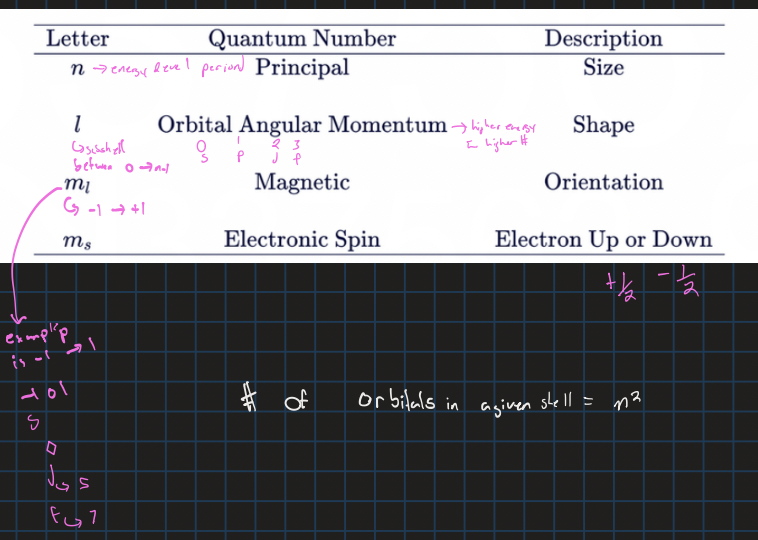
quantum numbers
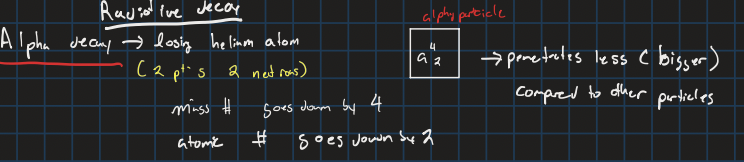
alpha decay
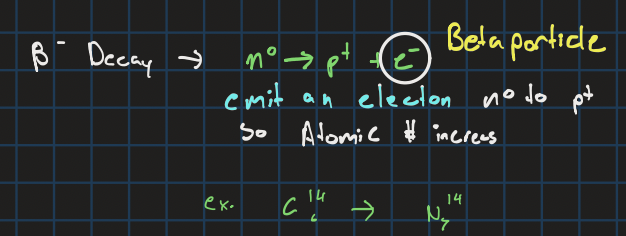
Beta- decay
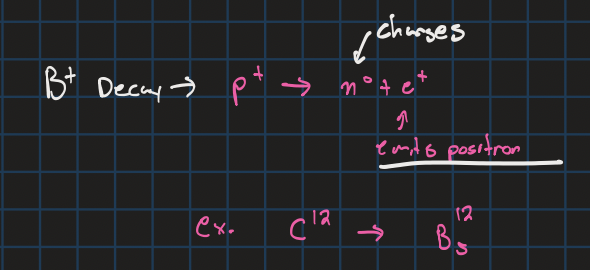
Beta+ decay
electron capture
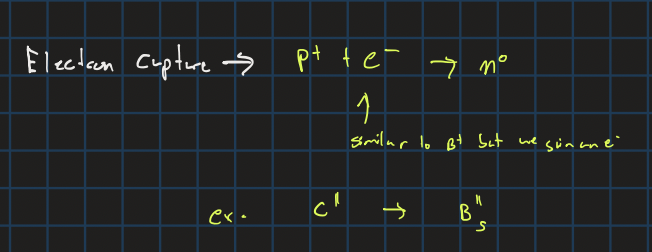
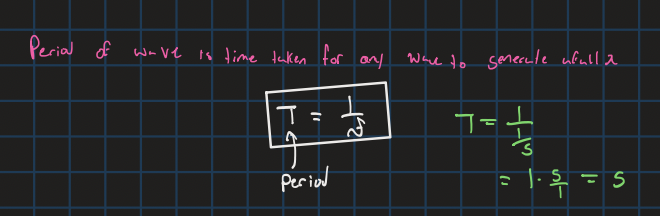
period
Speed of a sound wave related to stiffness and density
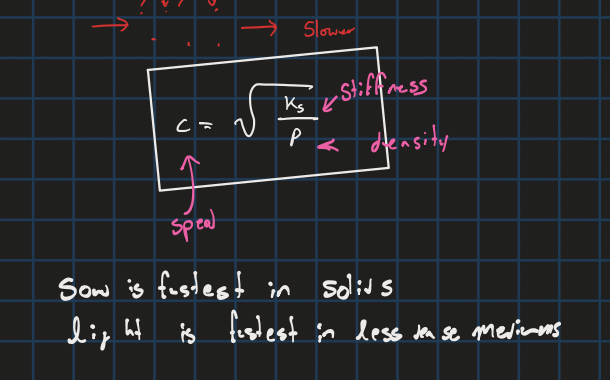
Speed of sound in air
343m/s
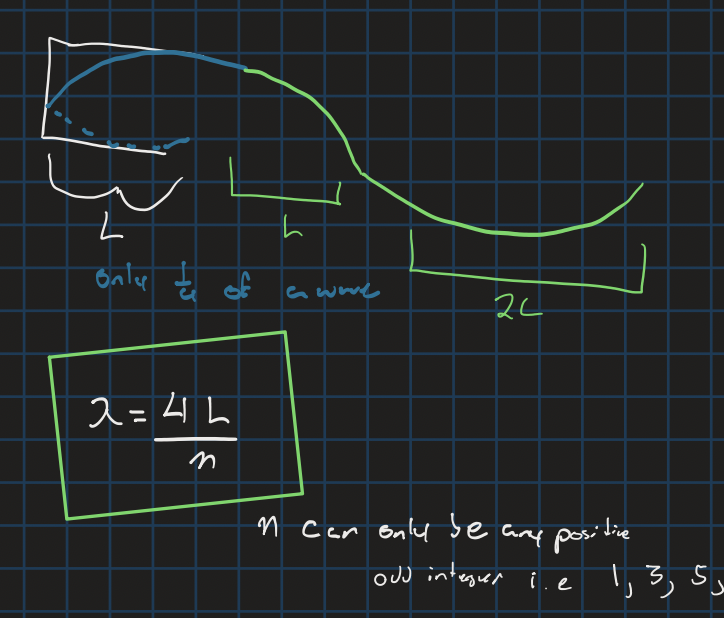
Harmonics with two open ends or two closed ends
n = any number 1 - x
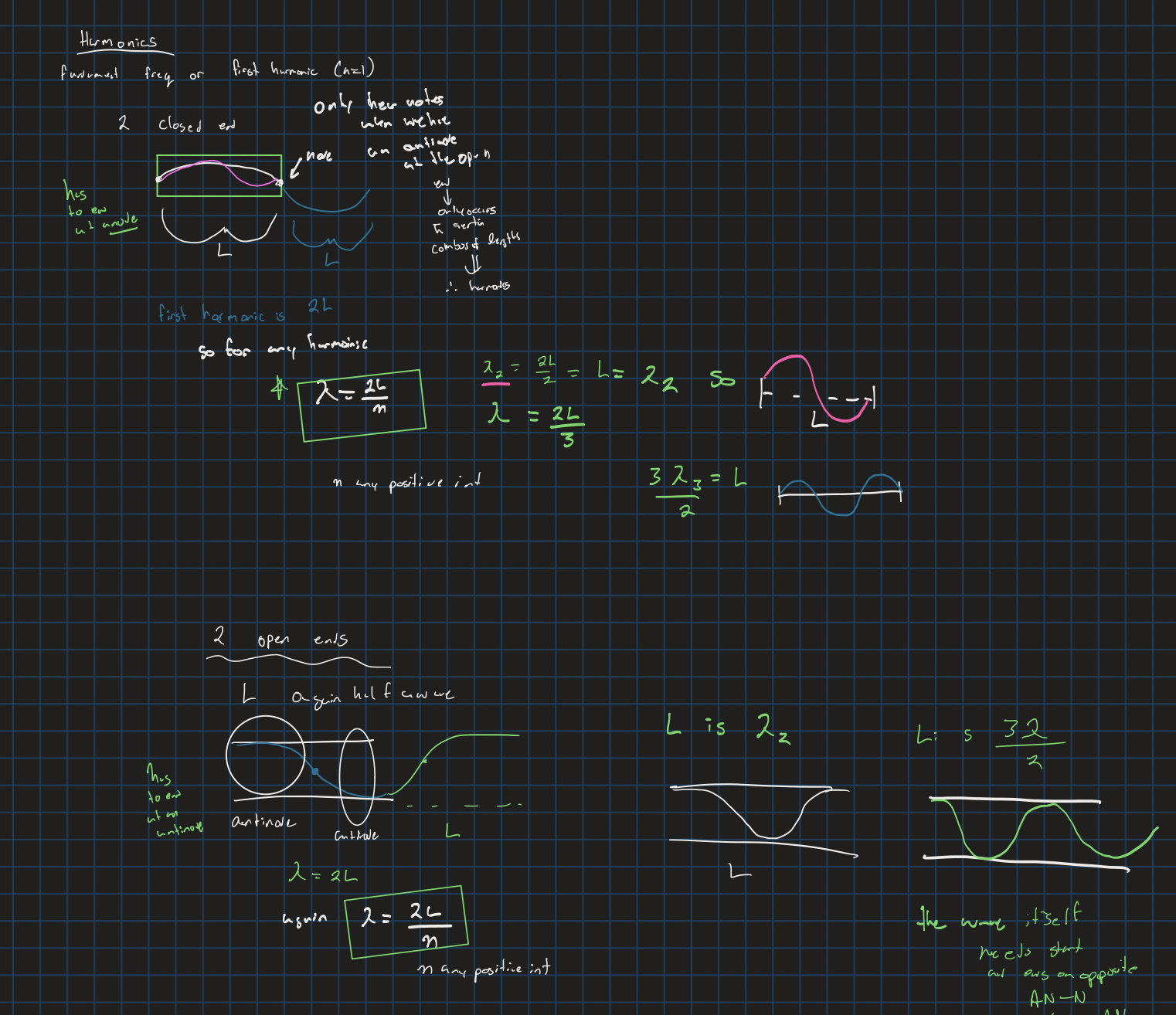
Harmonics with one open and one closed end
n is any positive odd integer (second is 3 and so on)
Sound intensity
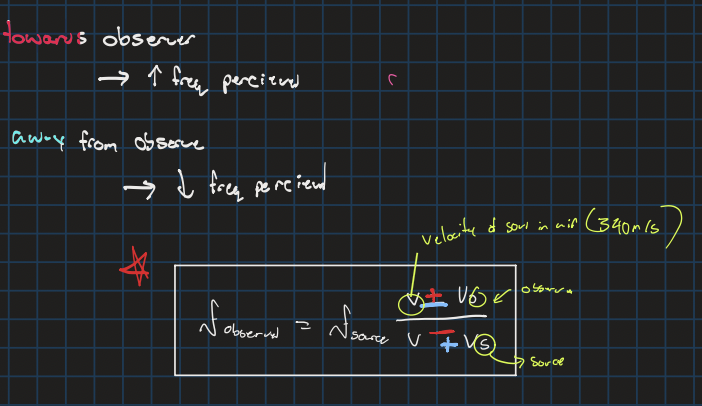
Dopler equation
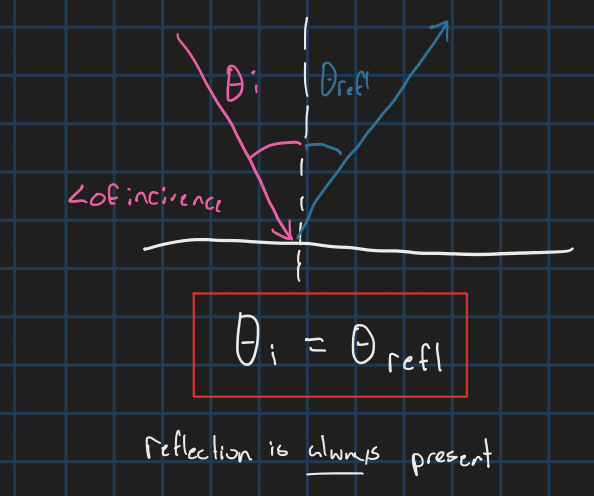
Reflection equation
index of refraction
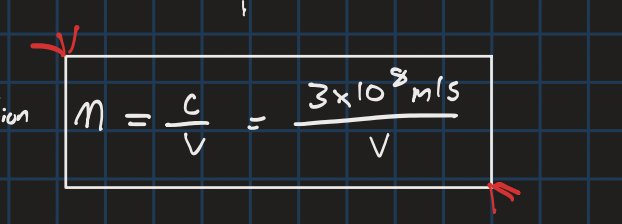
Snell’s law
to derive angle of refraction or the speed
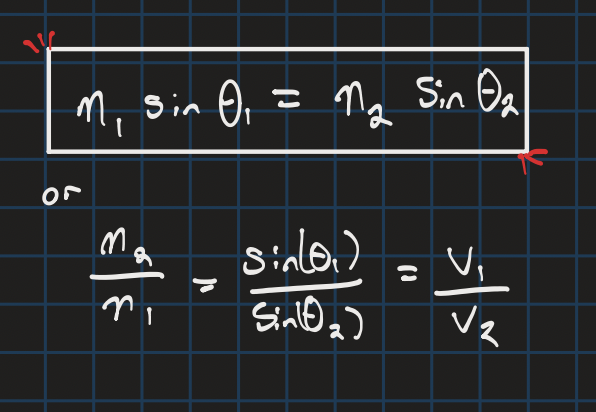
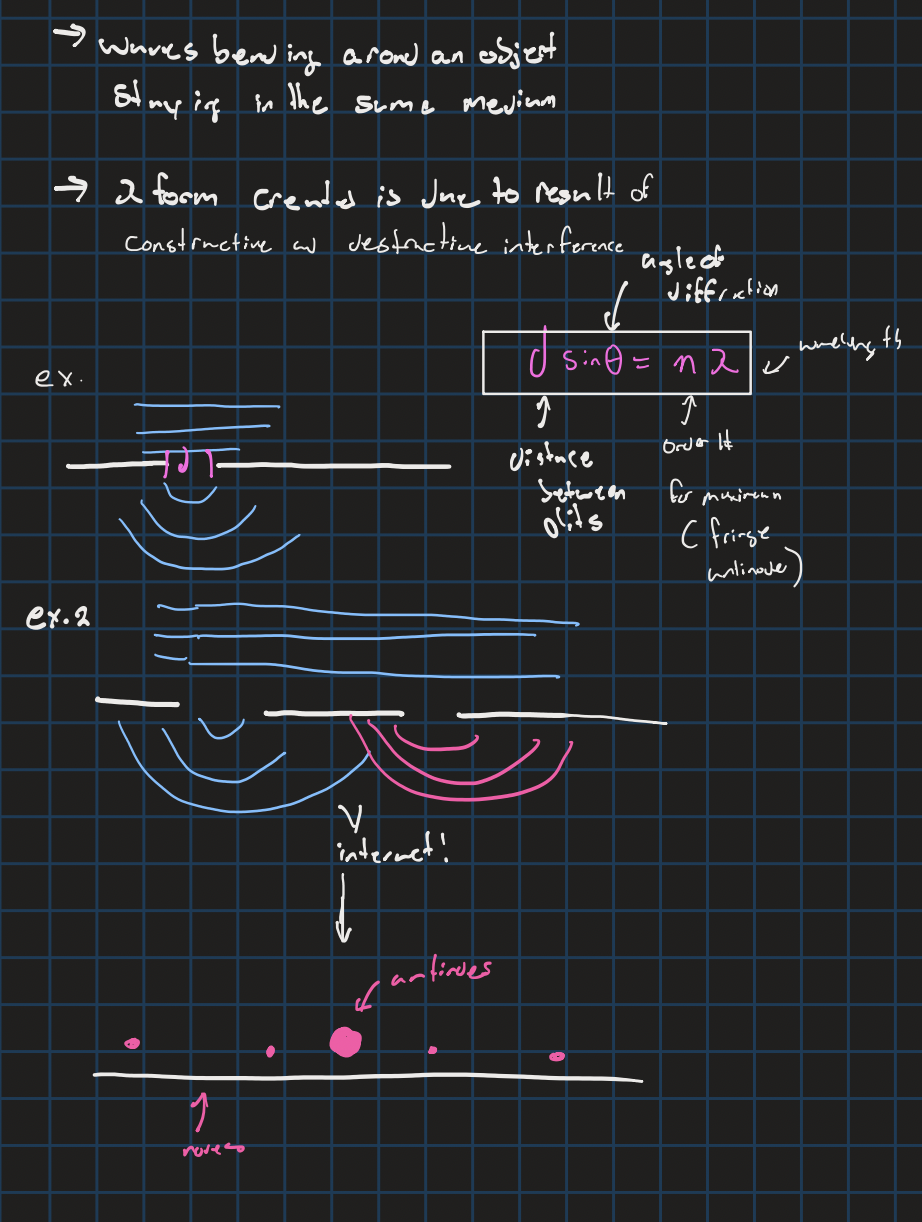
diffraction equation
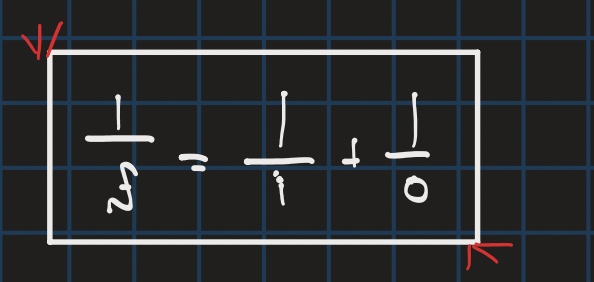
lensmakers equation
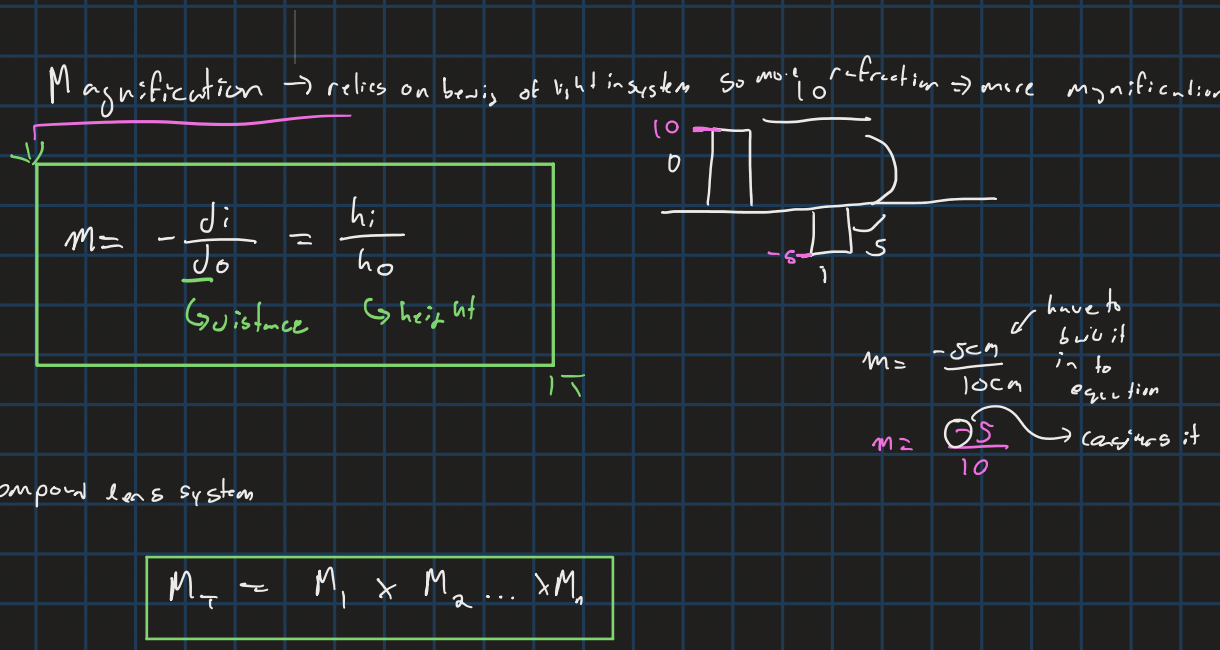
magnification
Power of a lens/mirror
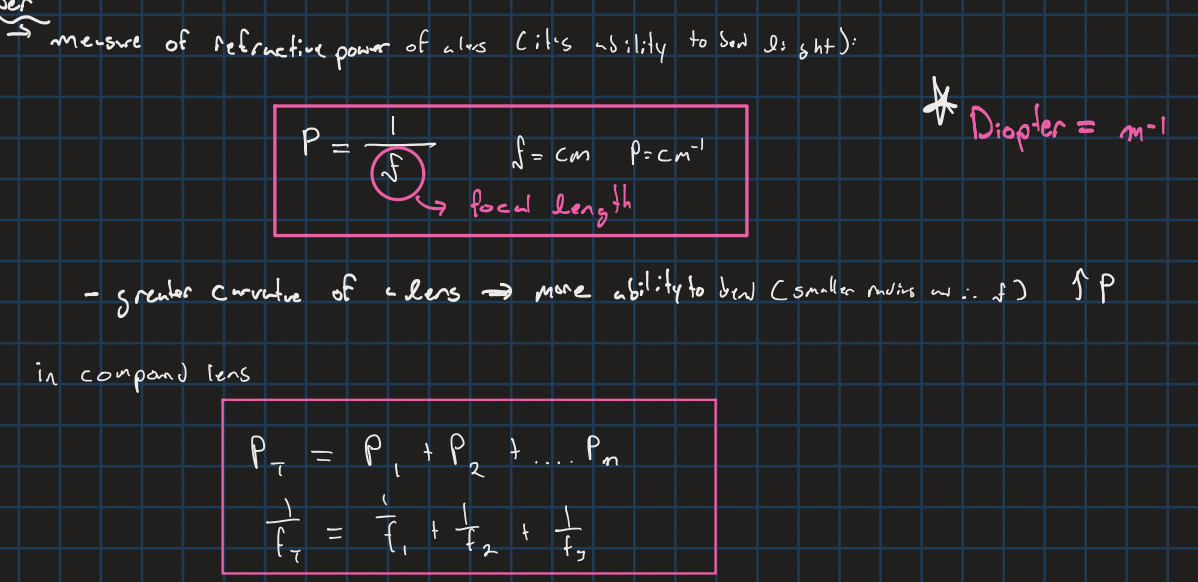
mass percent
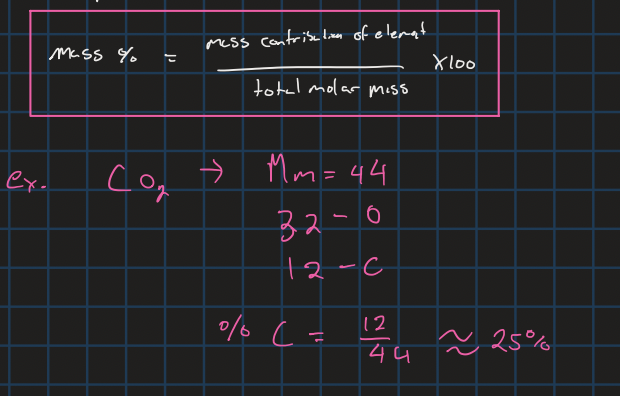
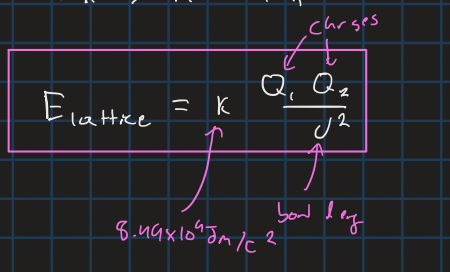
lattice energy
Formal charge
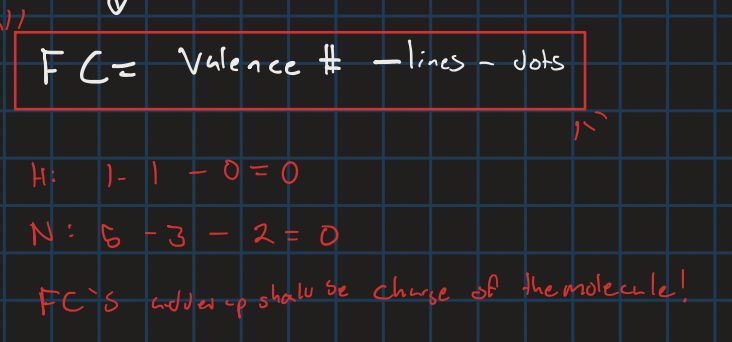
Charge of an electron/proton + absolute charge
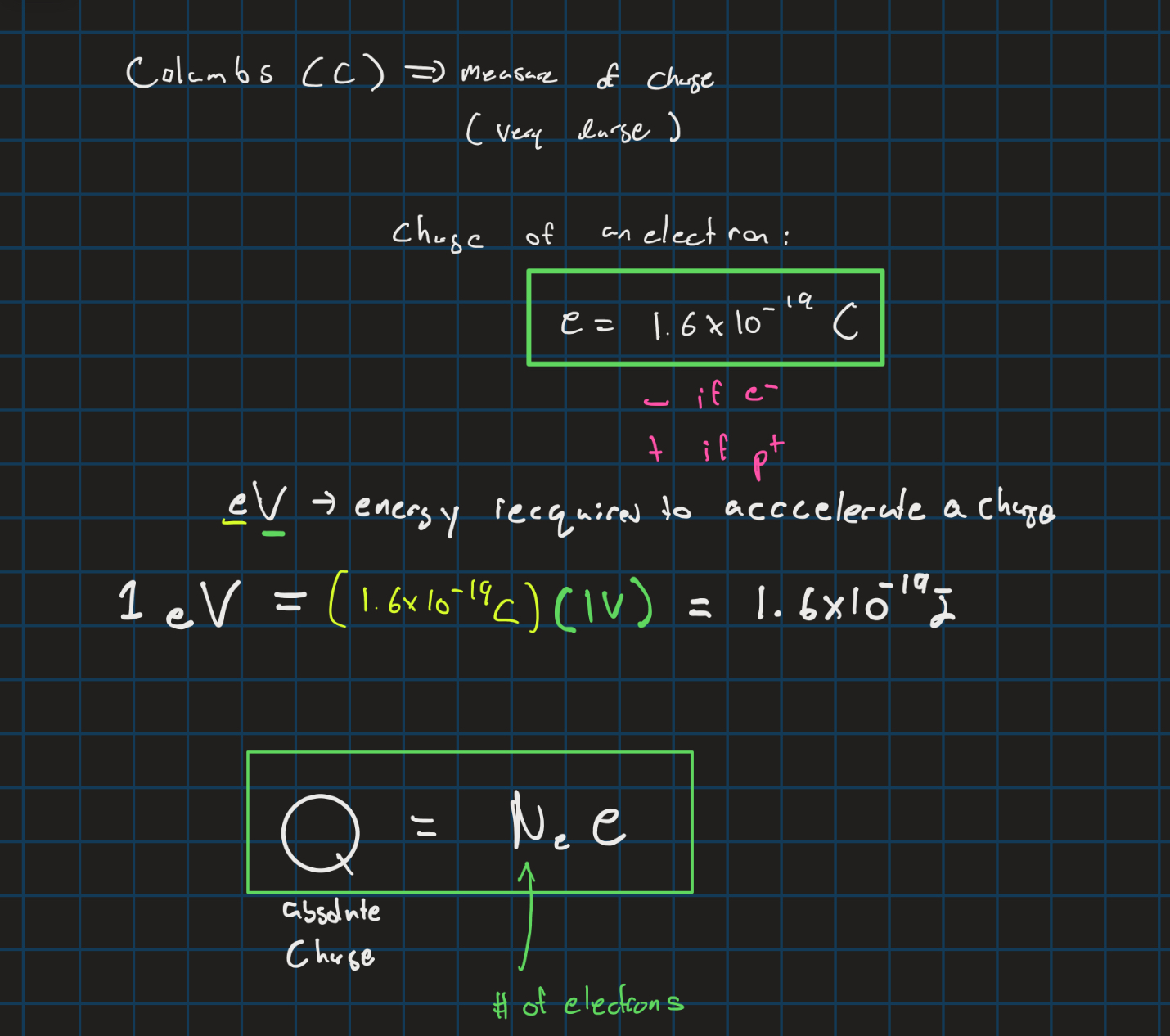
Electric field
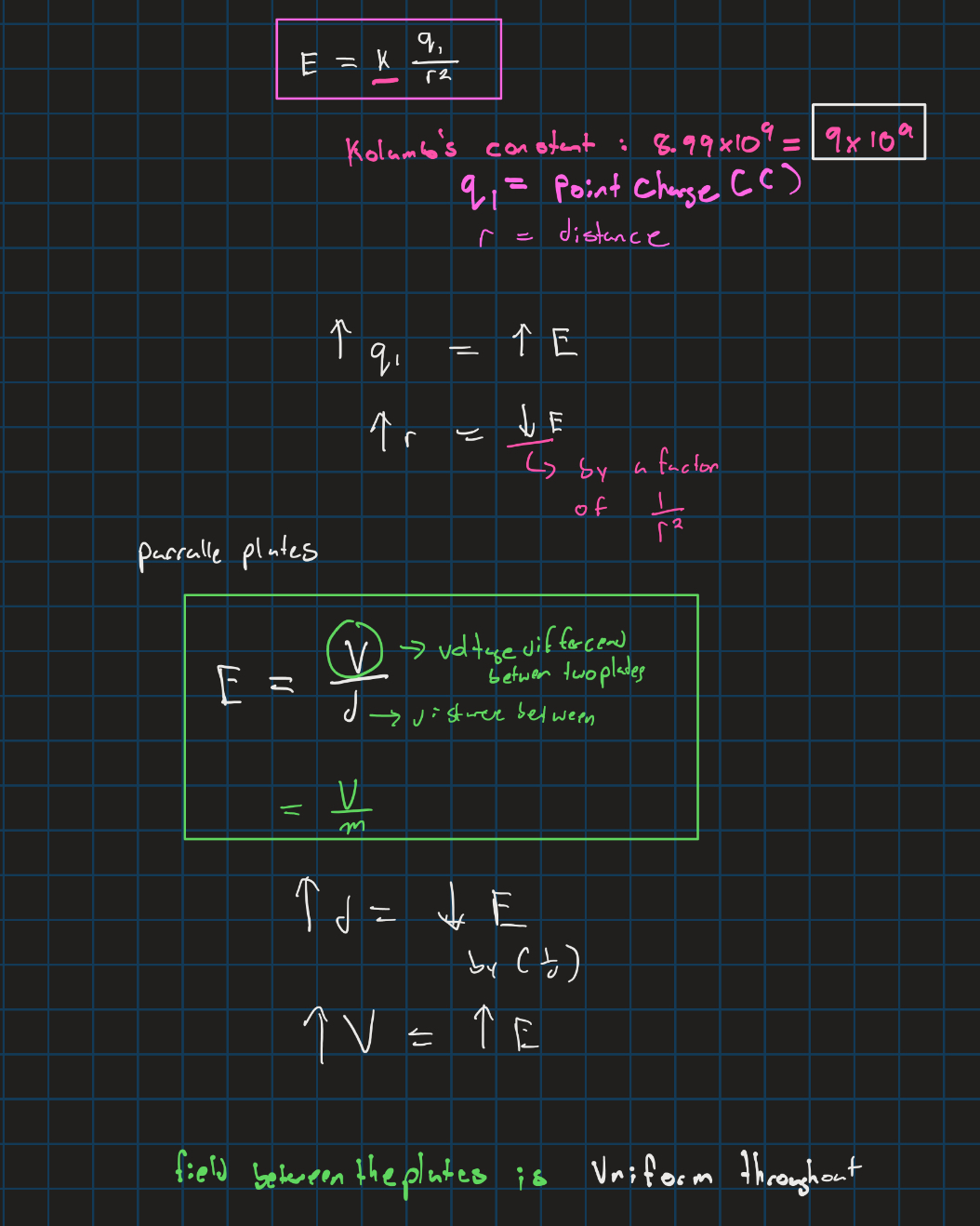
Gravitational constant
G = 6.67 X10-7
Columbs law and electric force
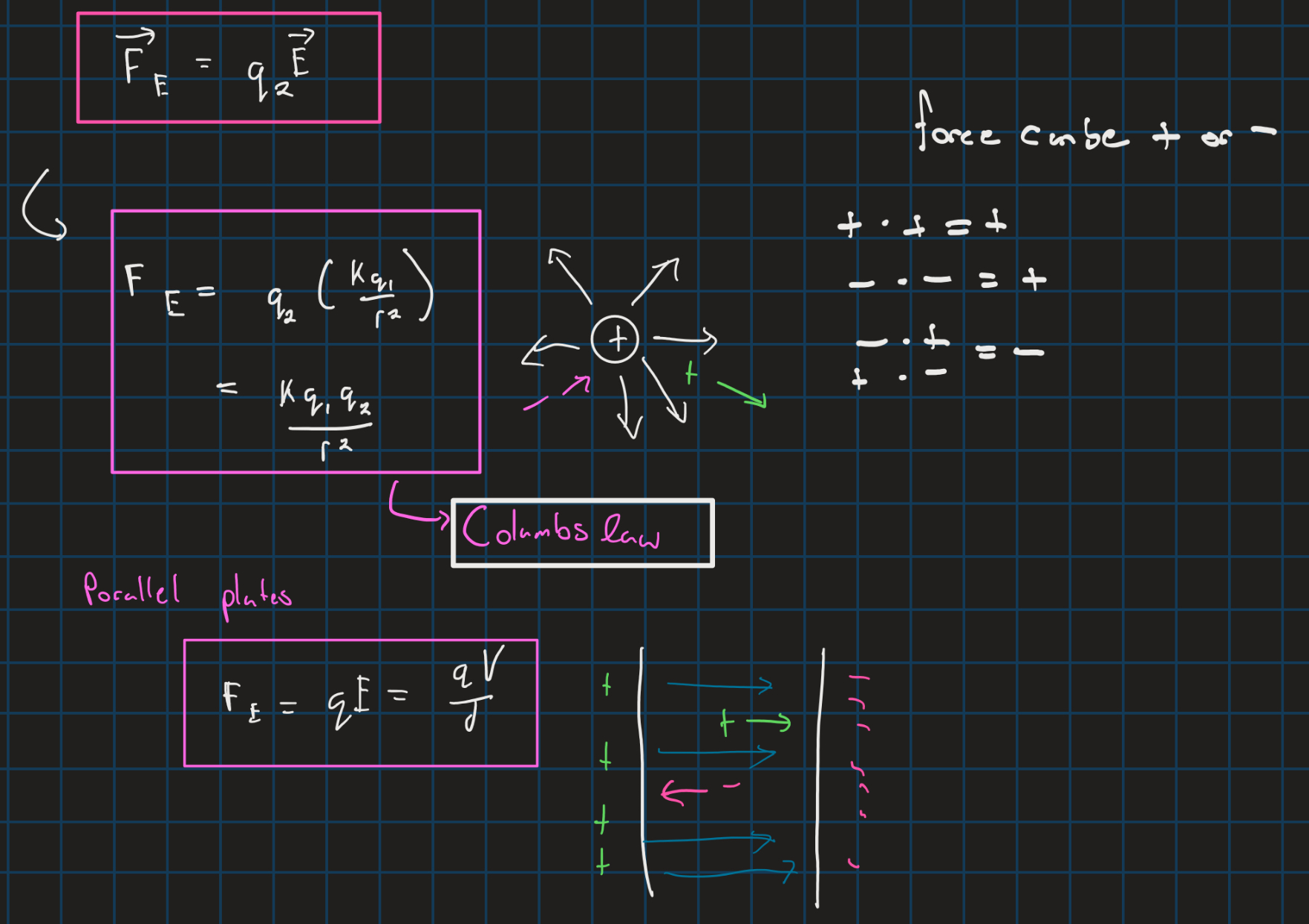
Electric potential
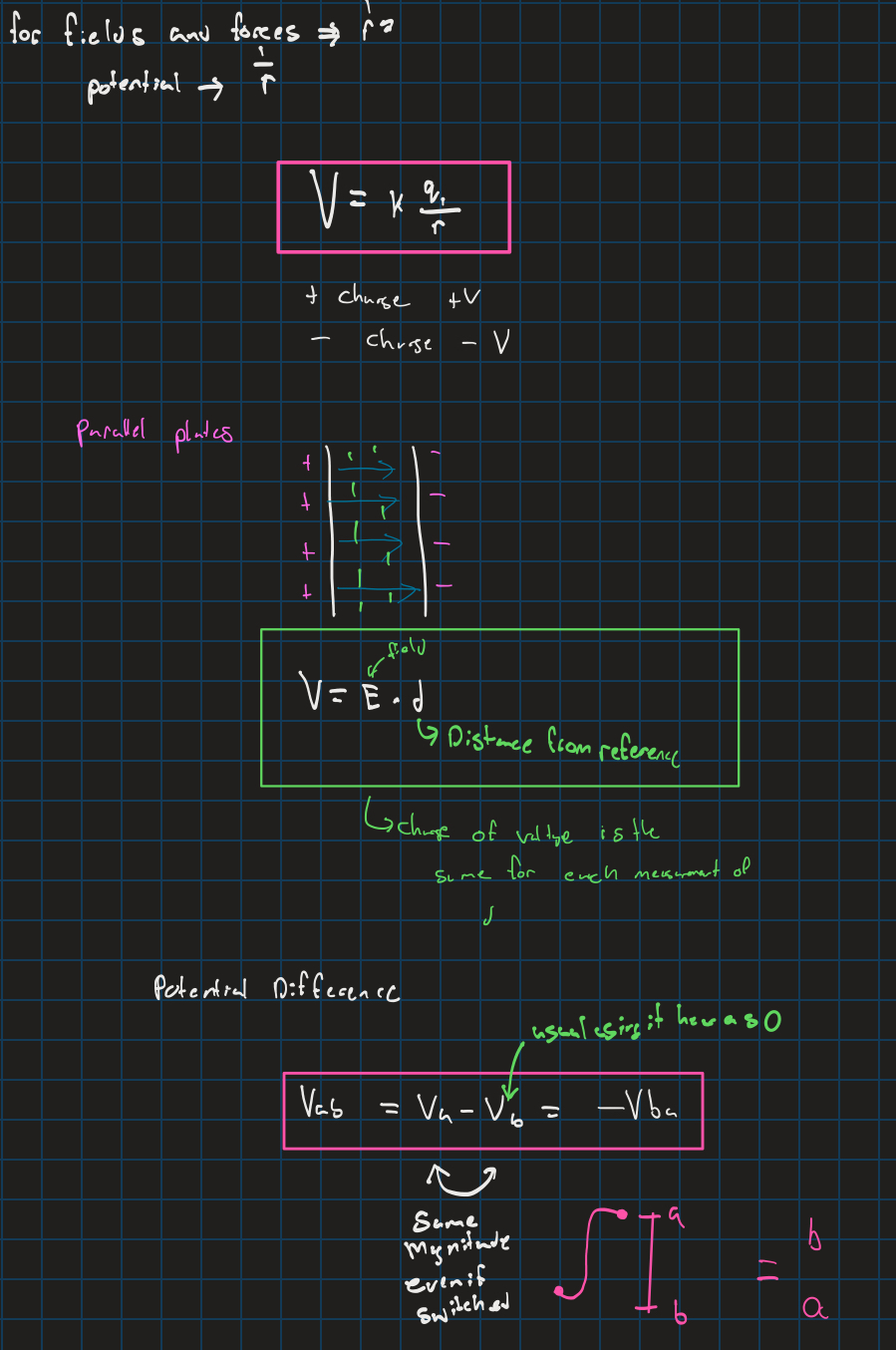
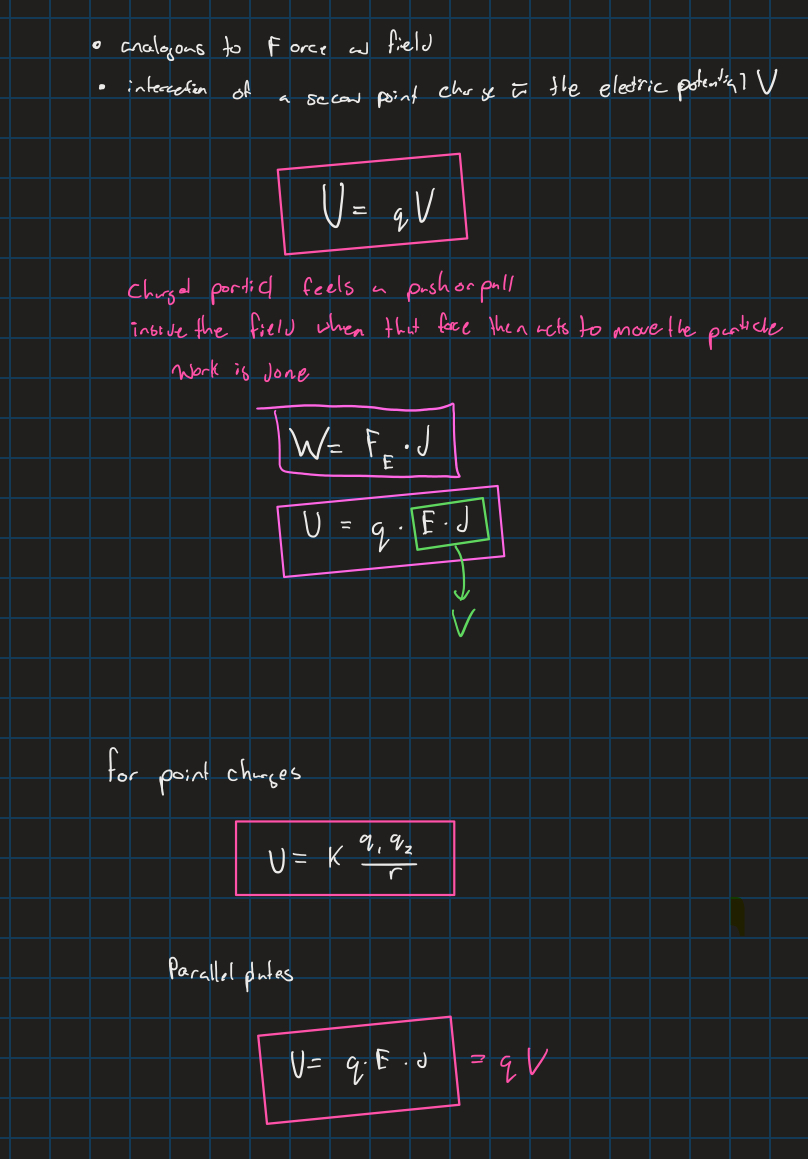
Electric potential energy (work)
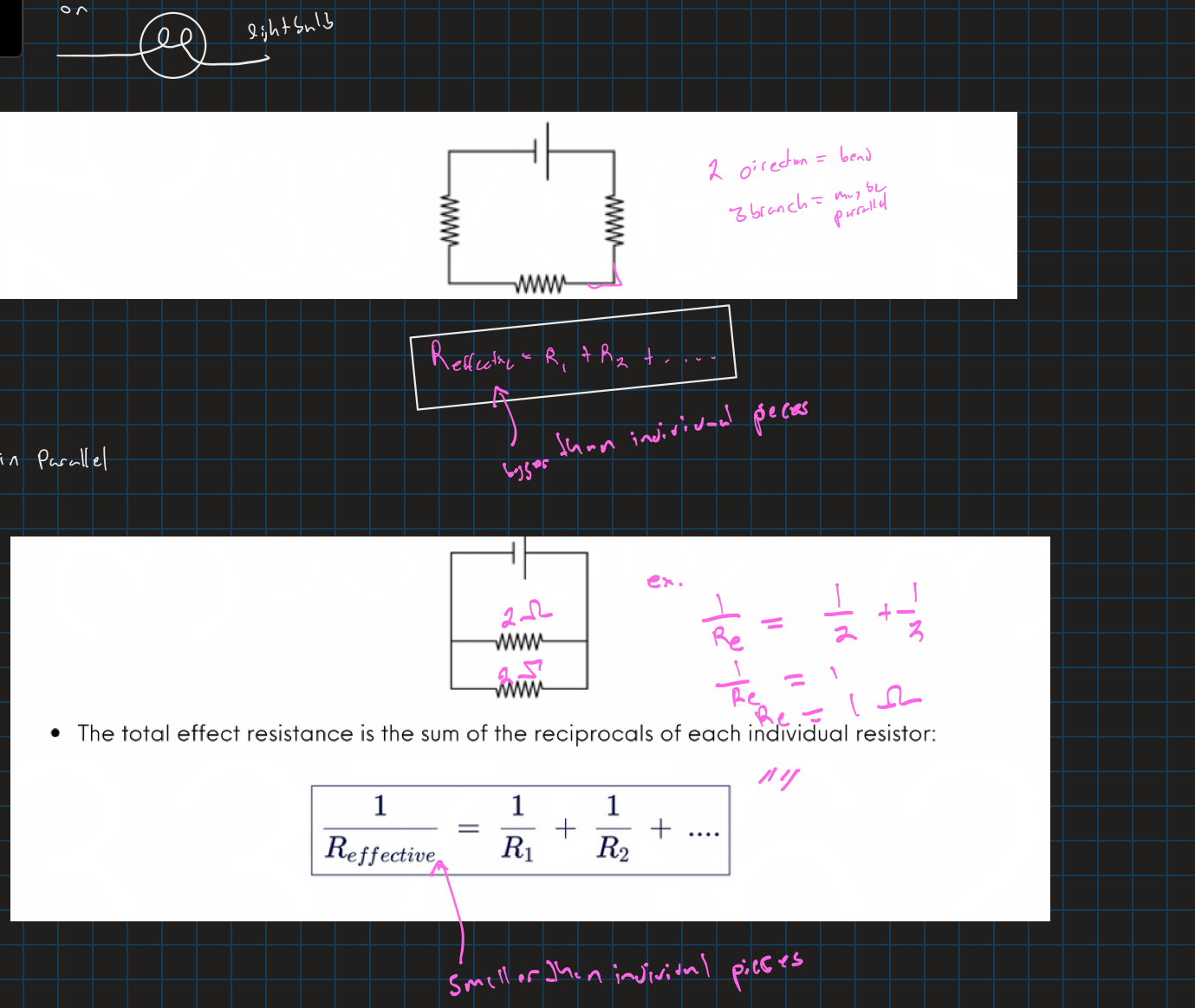
Resistors in series and parallel
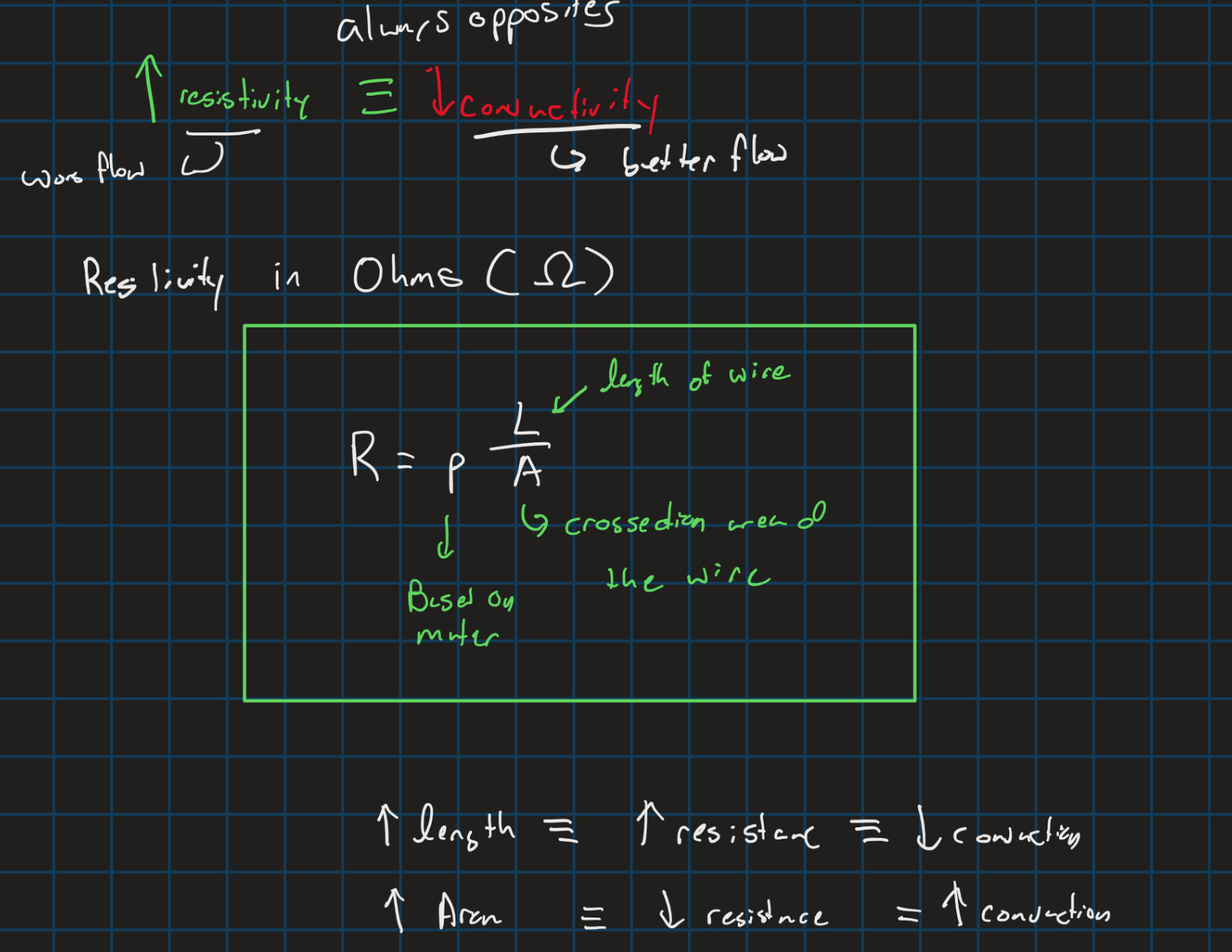
Resistance
Current
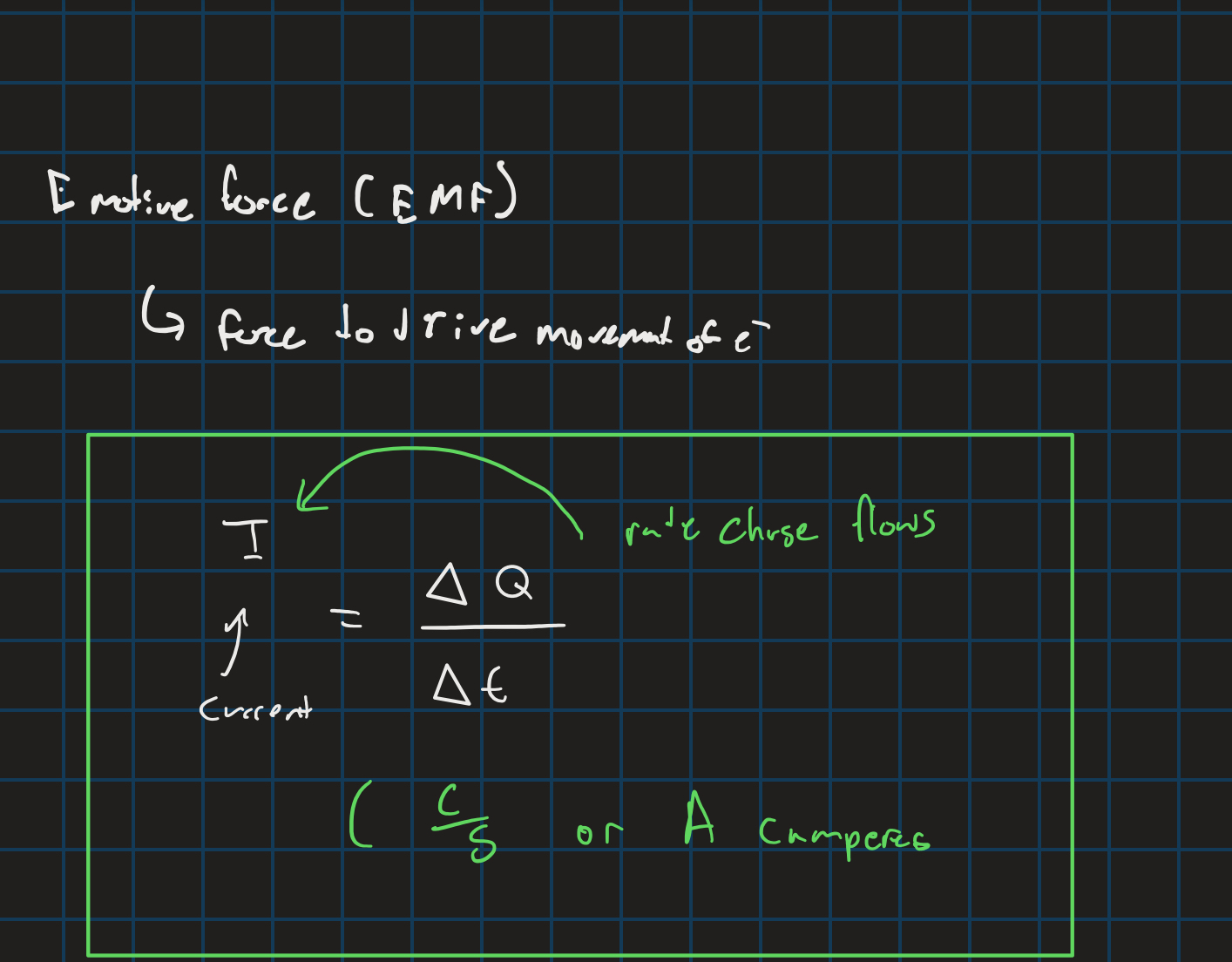
ohm’s law
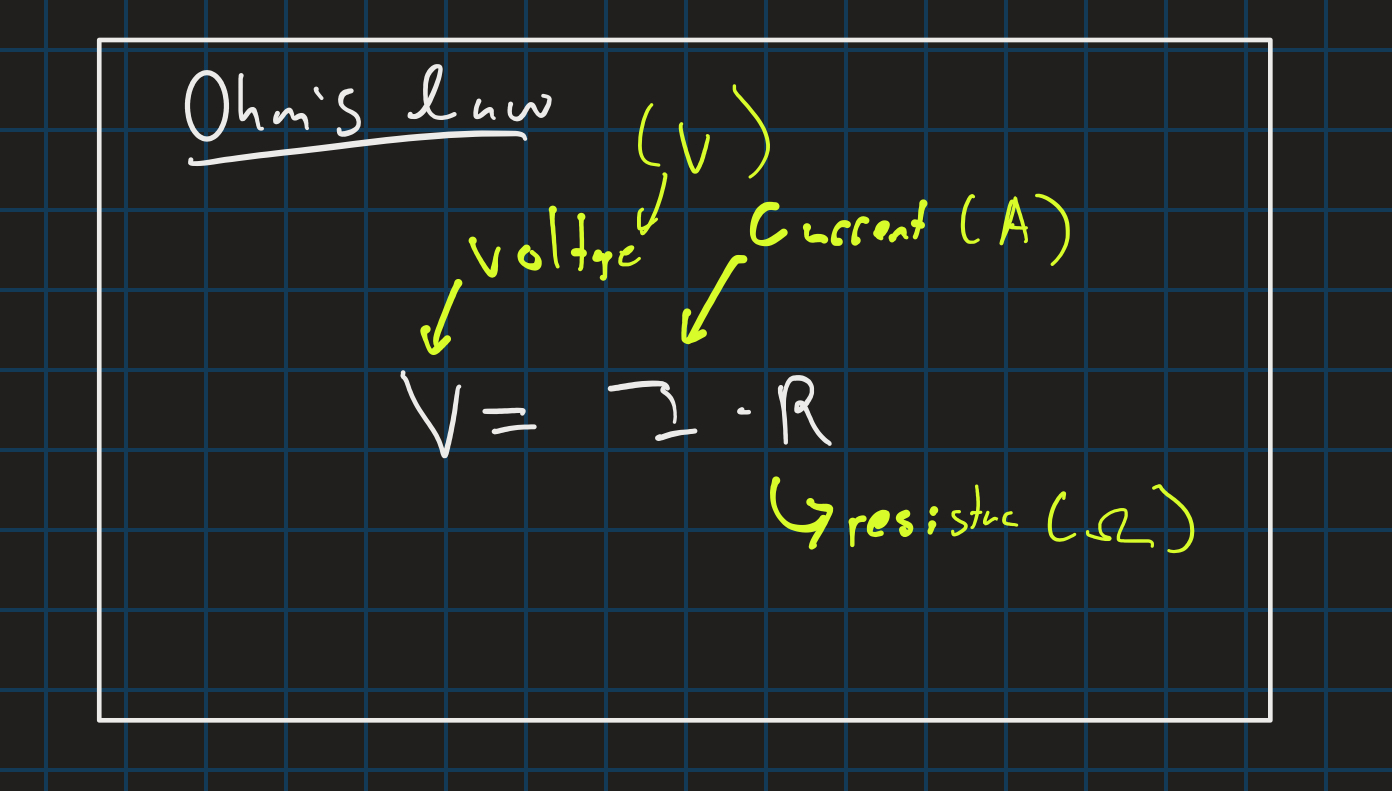
Power in circuits
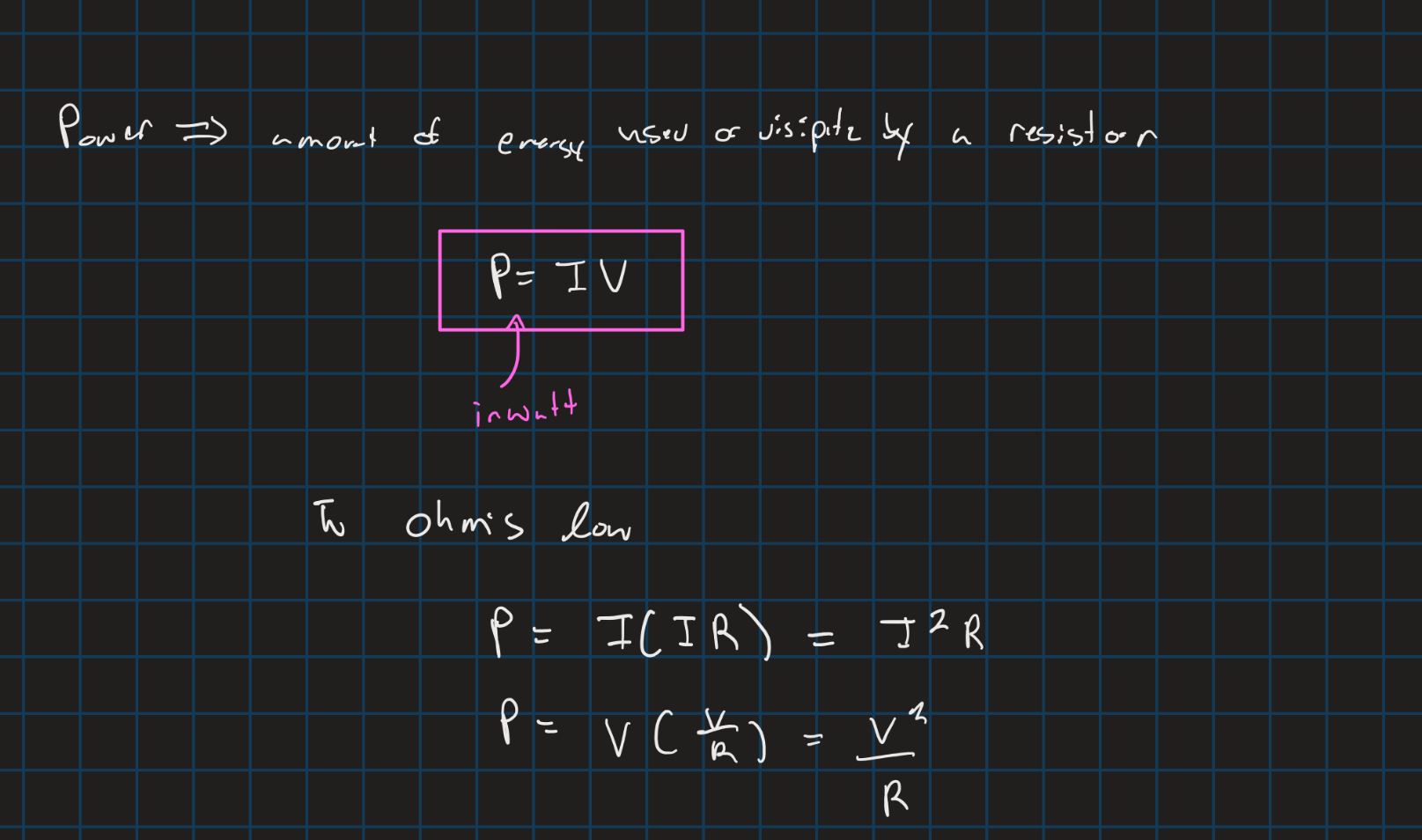
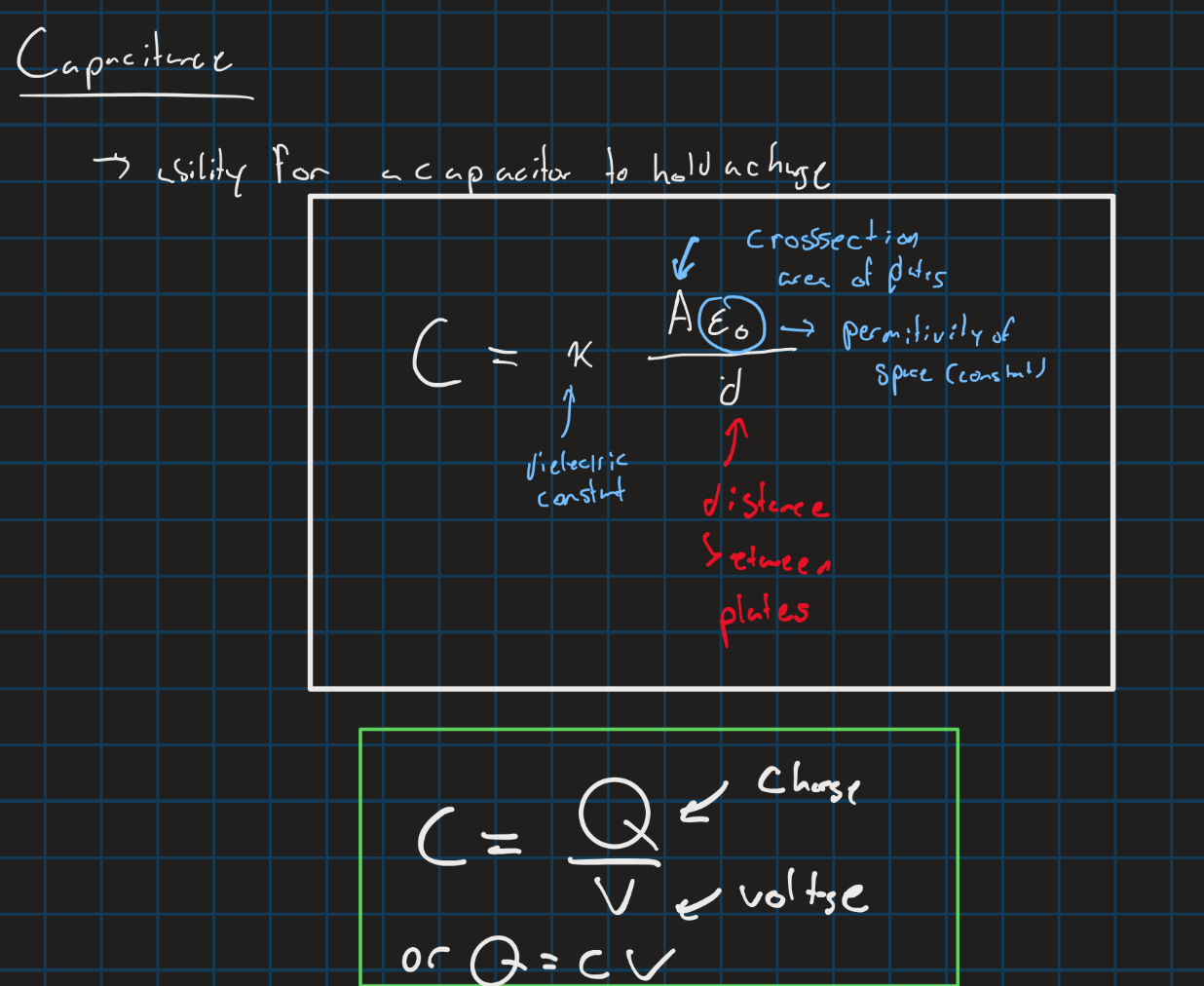
Capacitance
Electric potentially in a capacitor
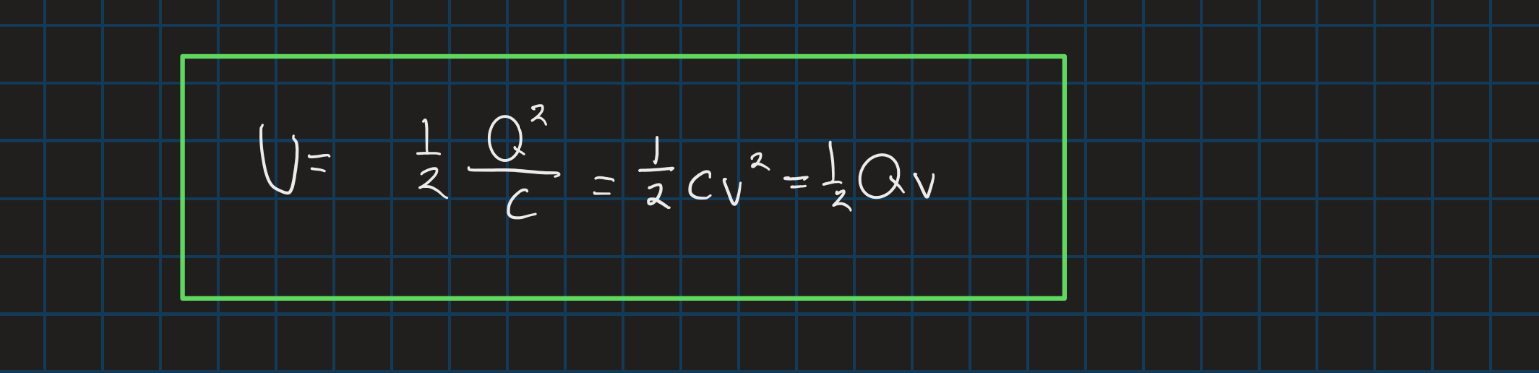
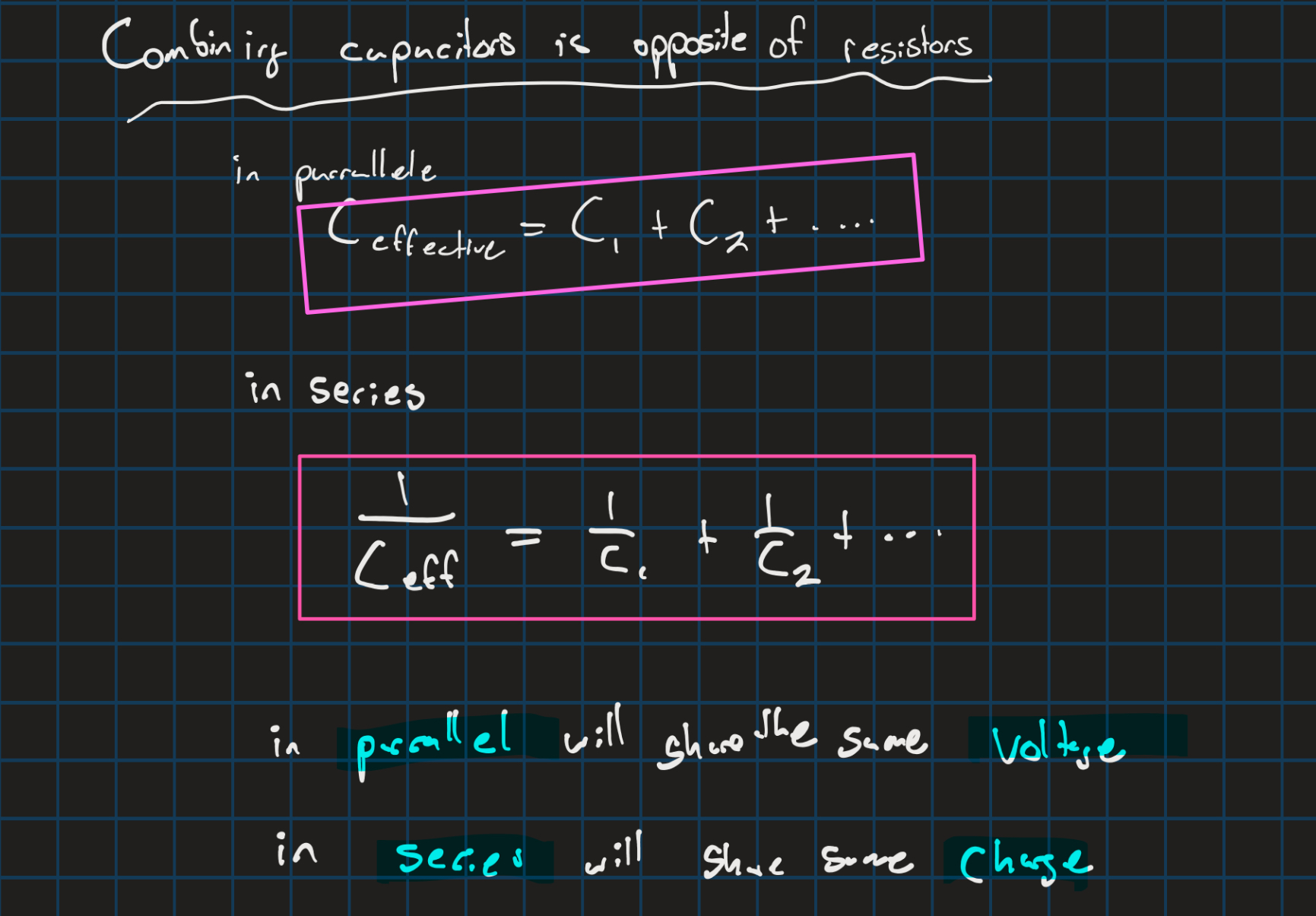
Capacitors in circuits
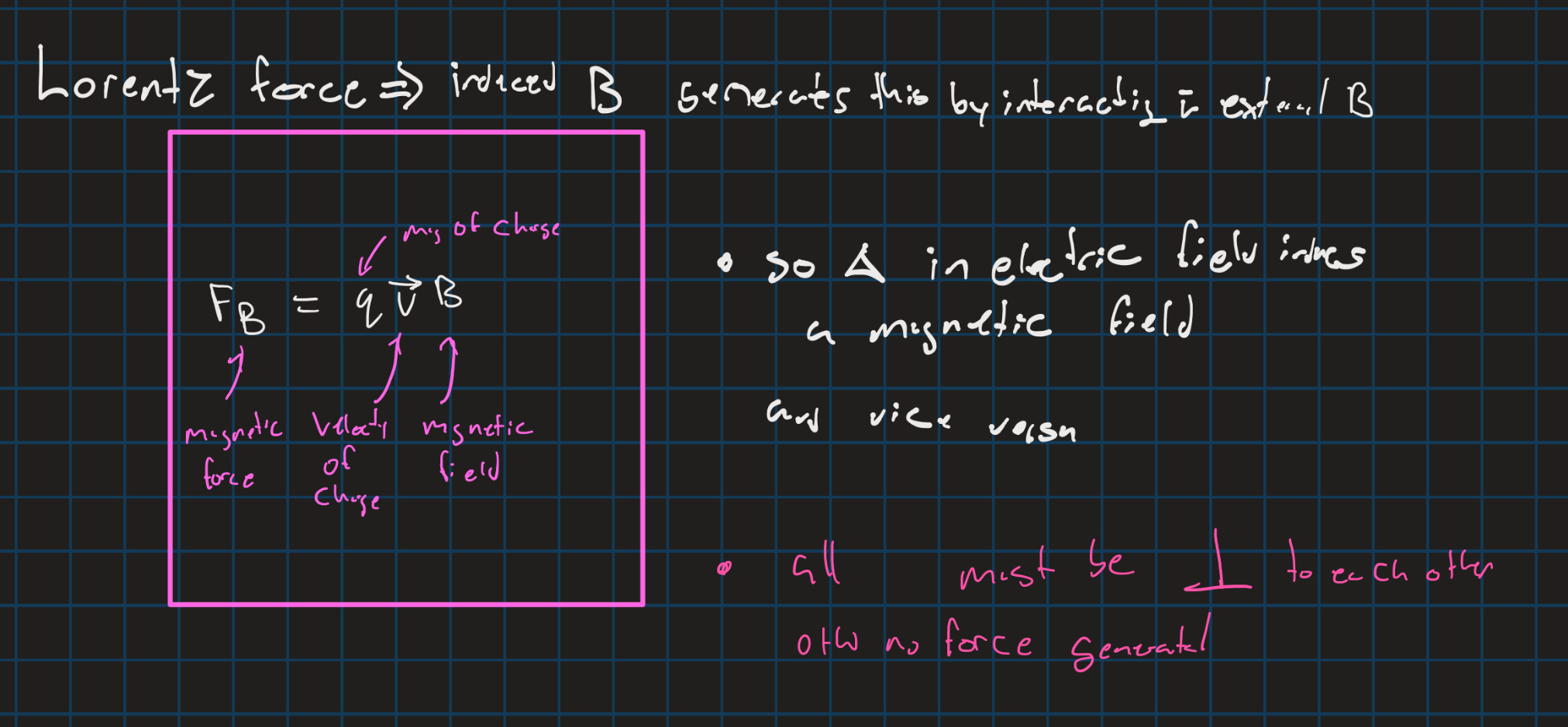
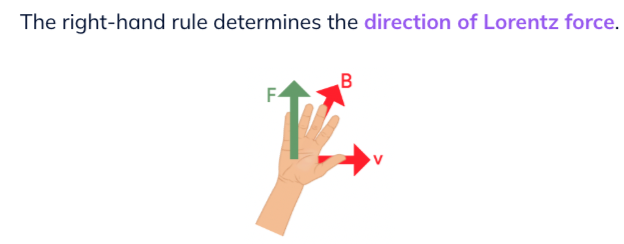
lorentz force
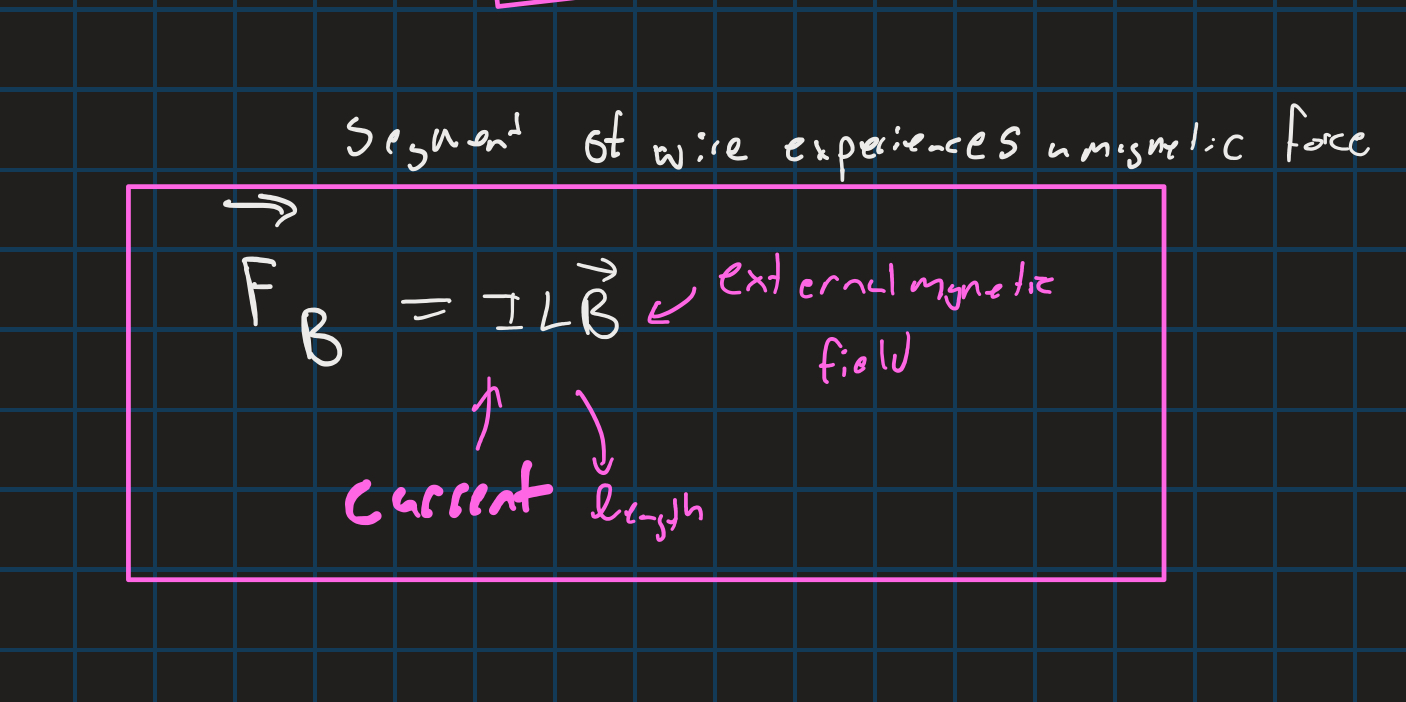
Magnetic force on a wire
Right hand rule
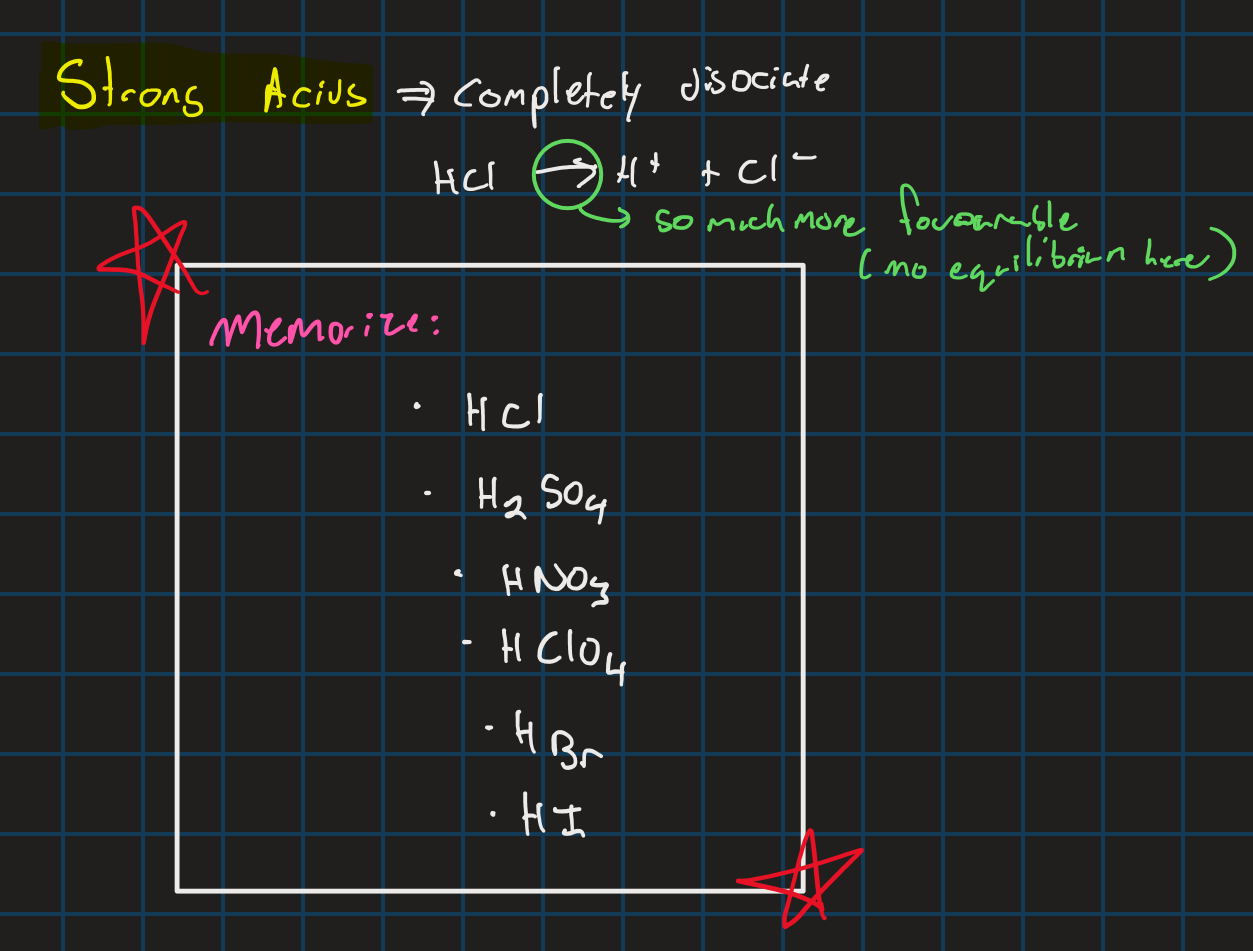
Strong acids
Ka and Pka
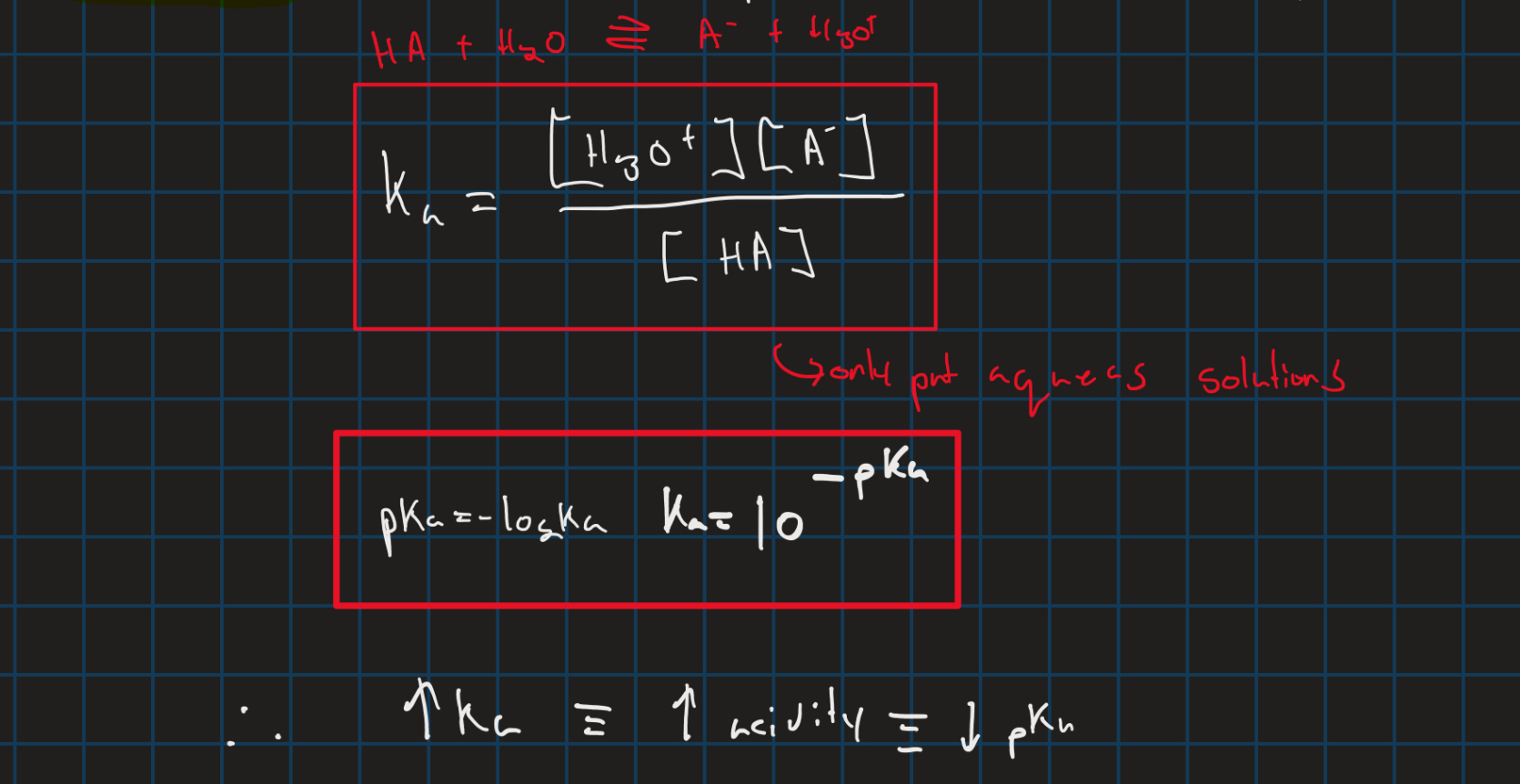
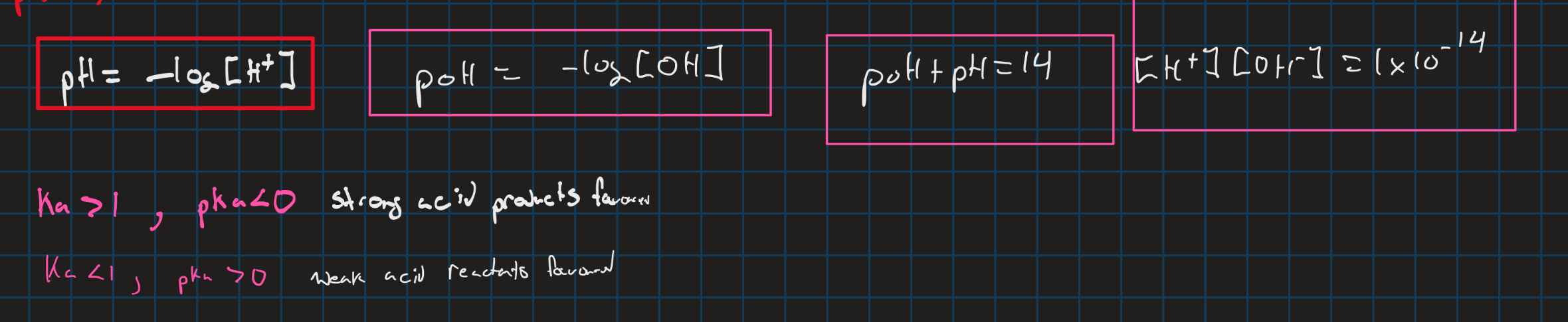
pH pOH and H+ and Oh- concentrations
Strong bases
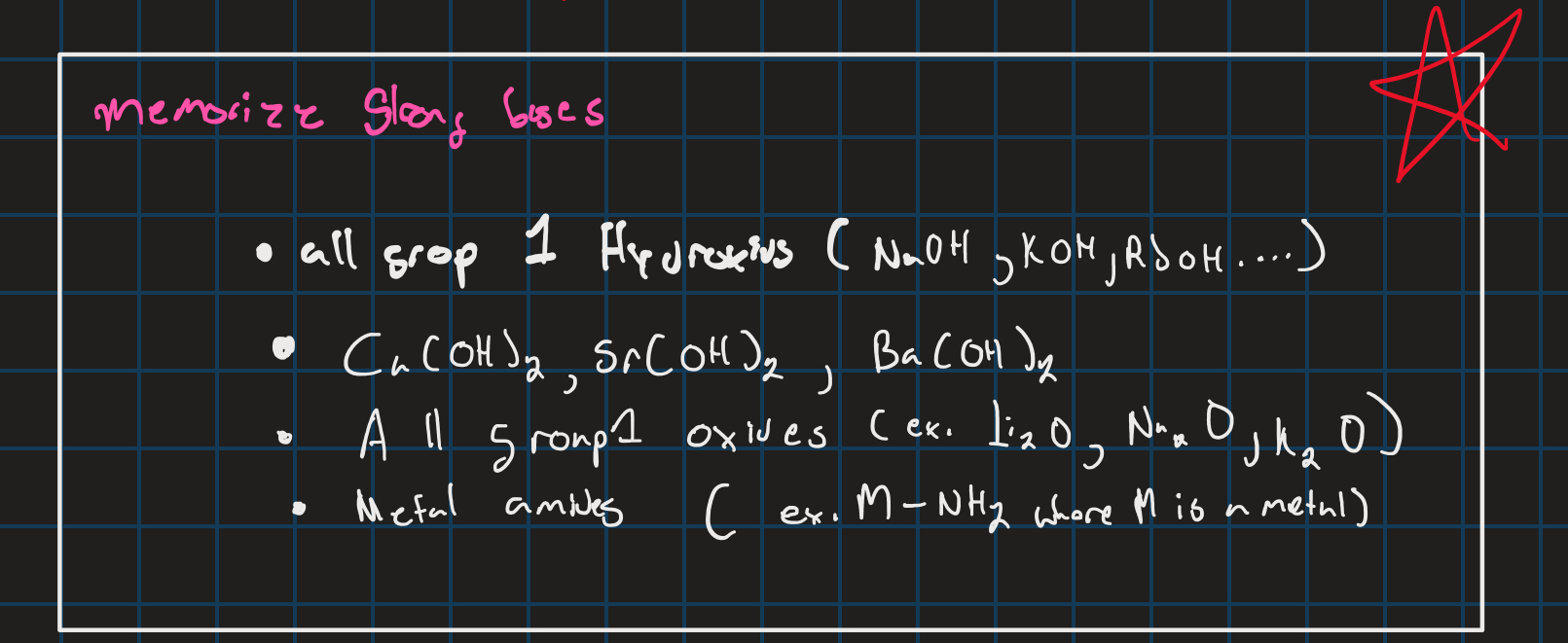
kb and pkb
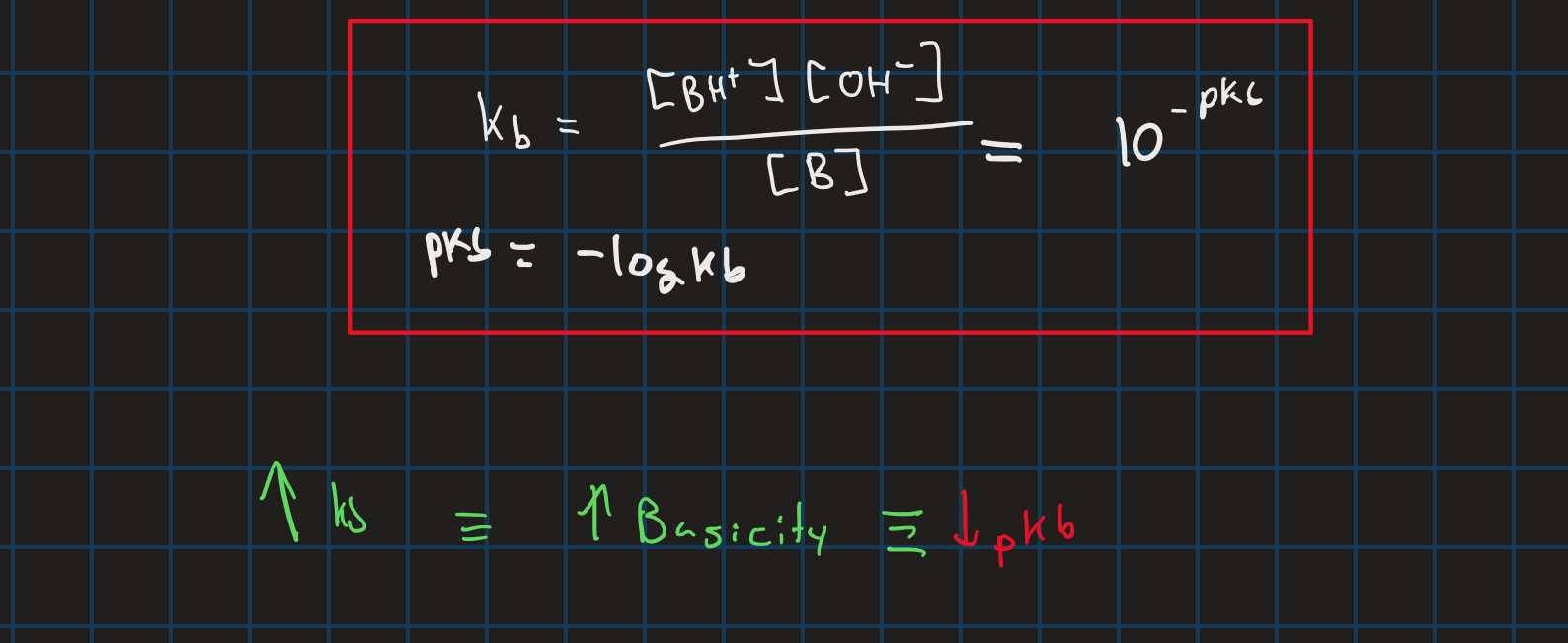
kw
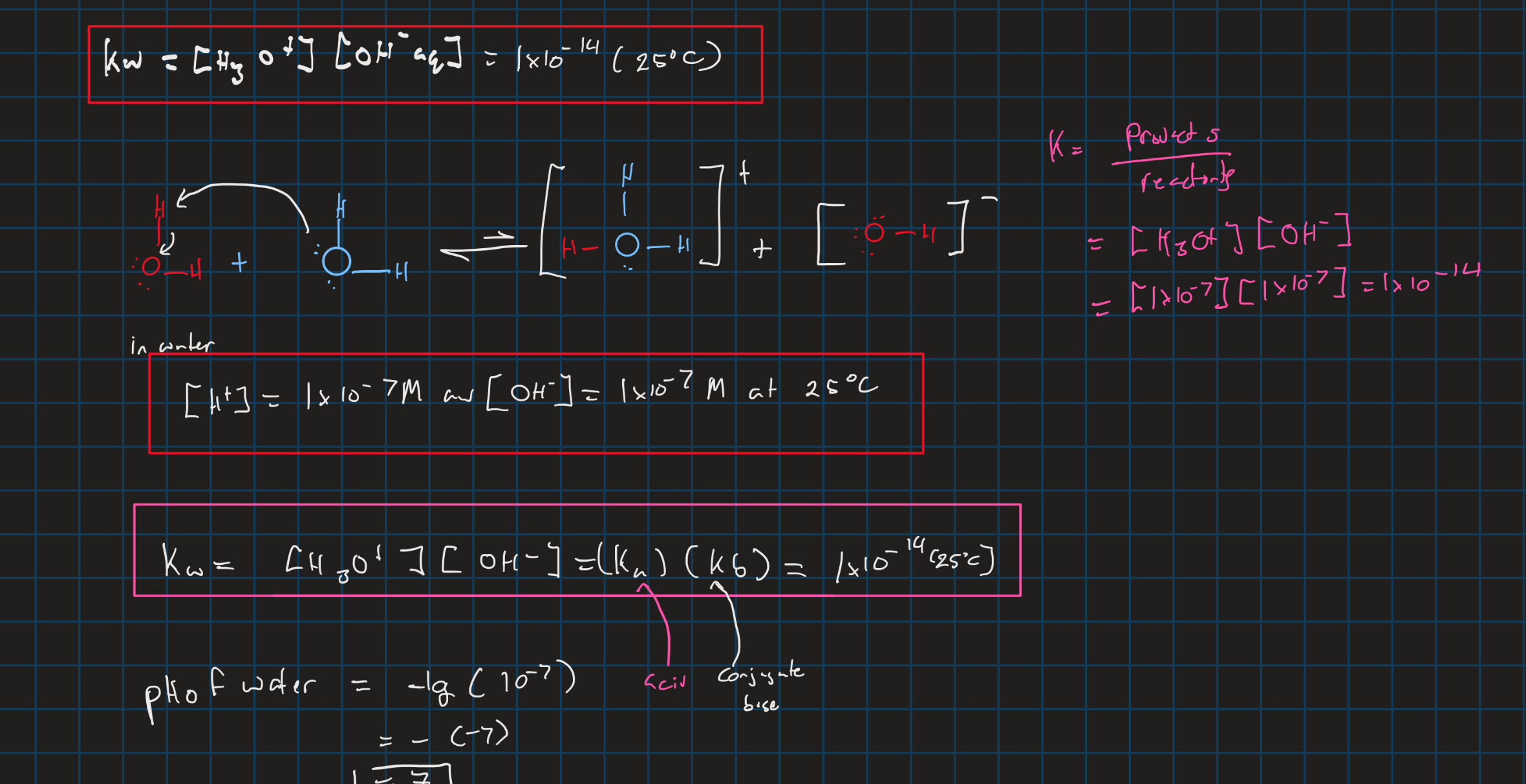
Henderson Hasselbach
use pka of acid to calculate pkb of it conjugate base and vice versa (same for pOH but with Pkb and BH+ /B)

Titration equation
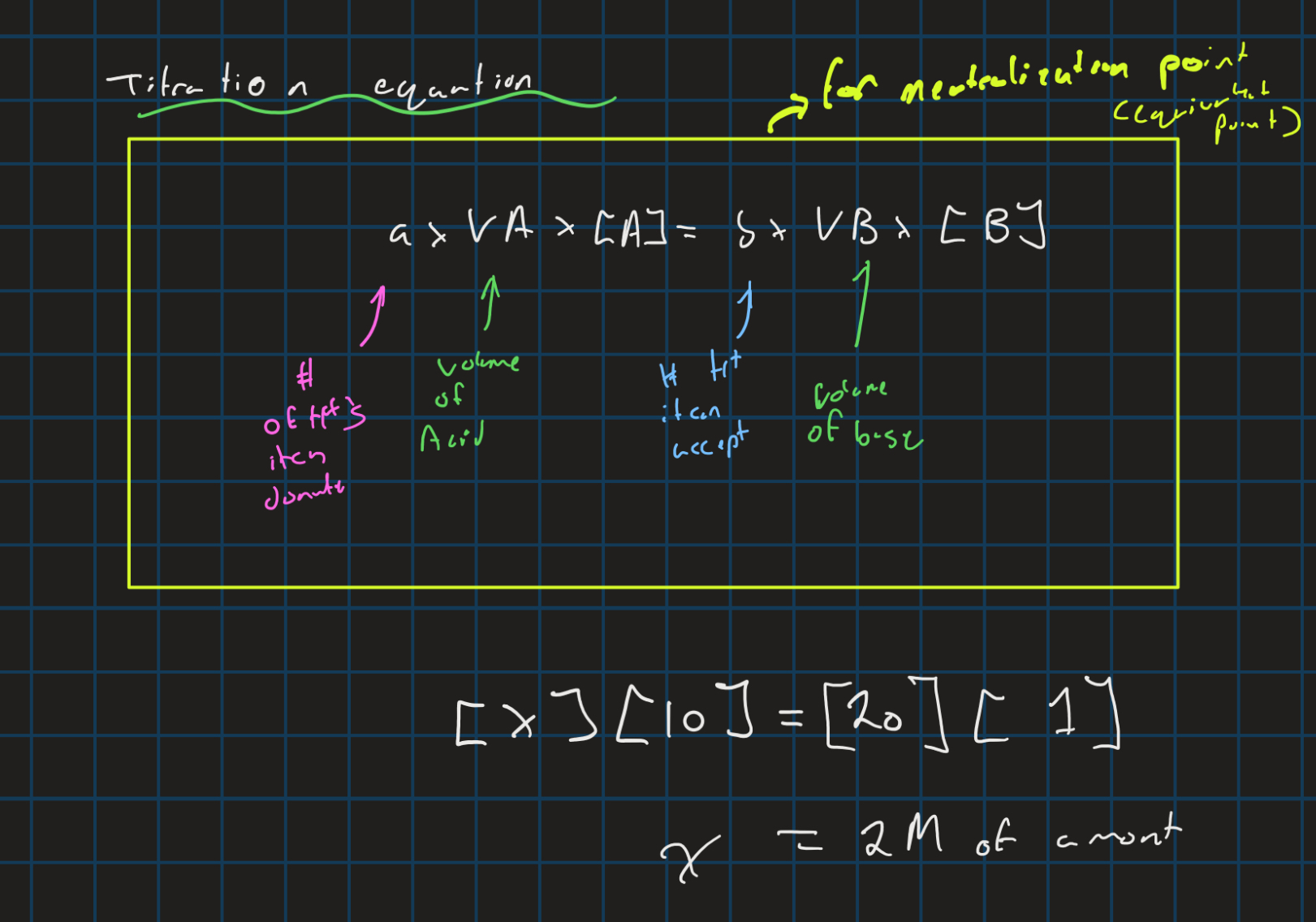
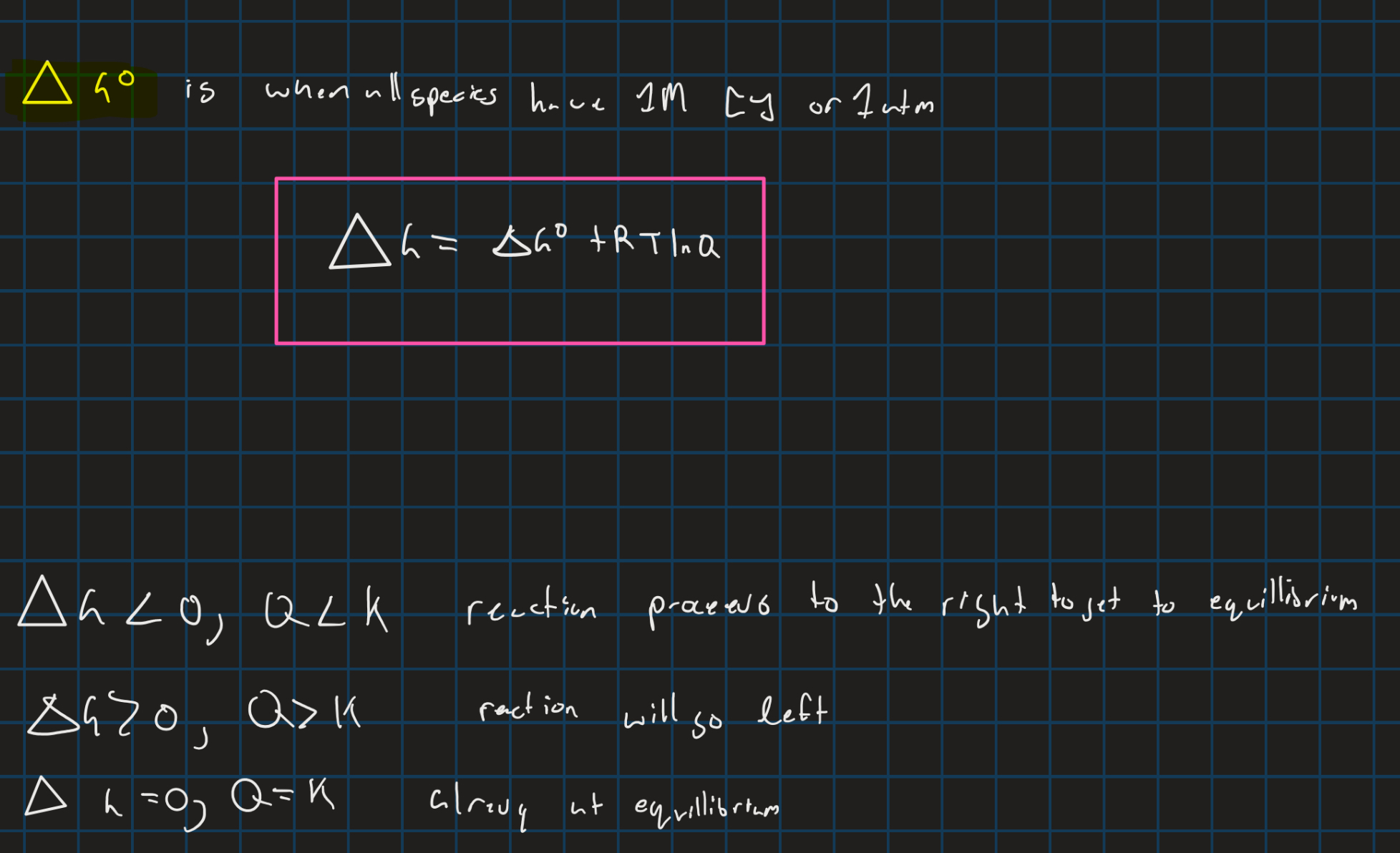
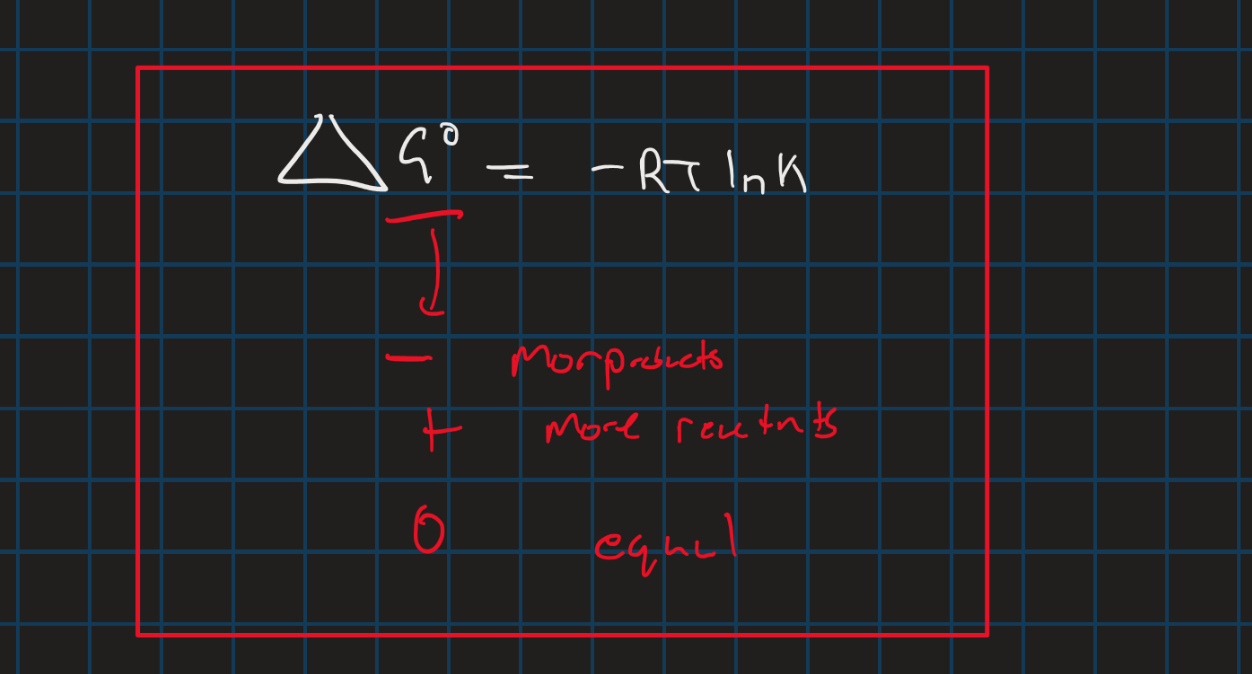
Equilibrium constant
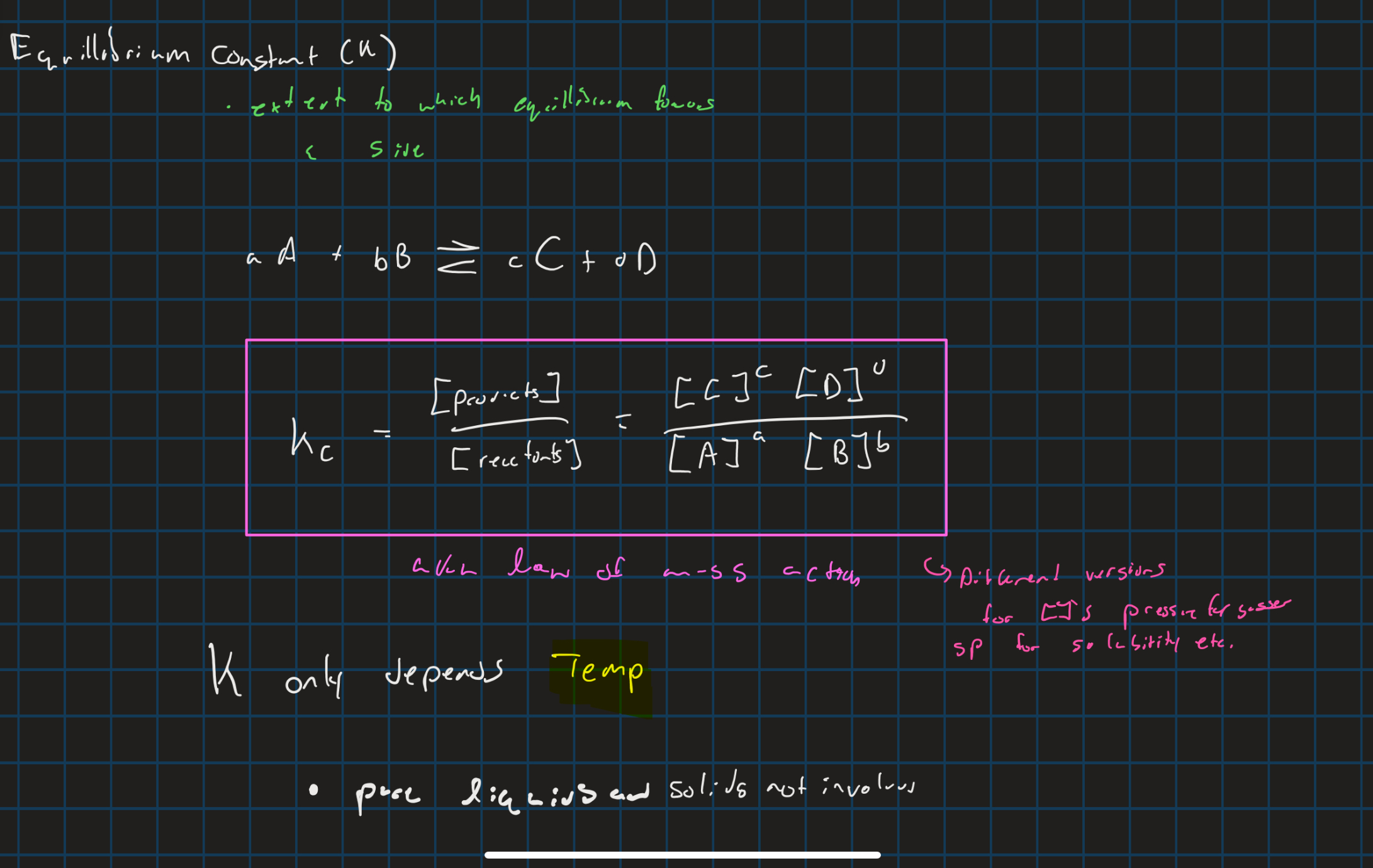
reaction quotient

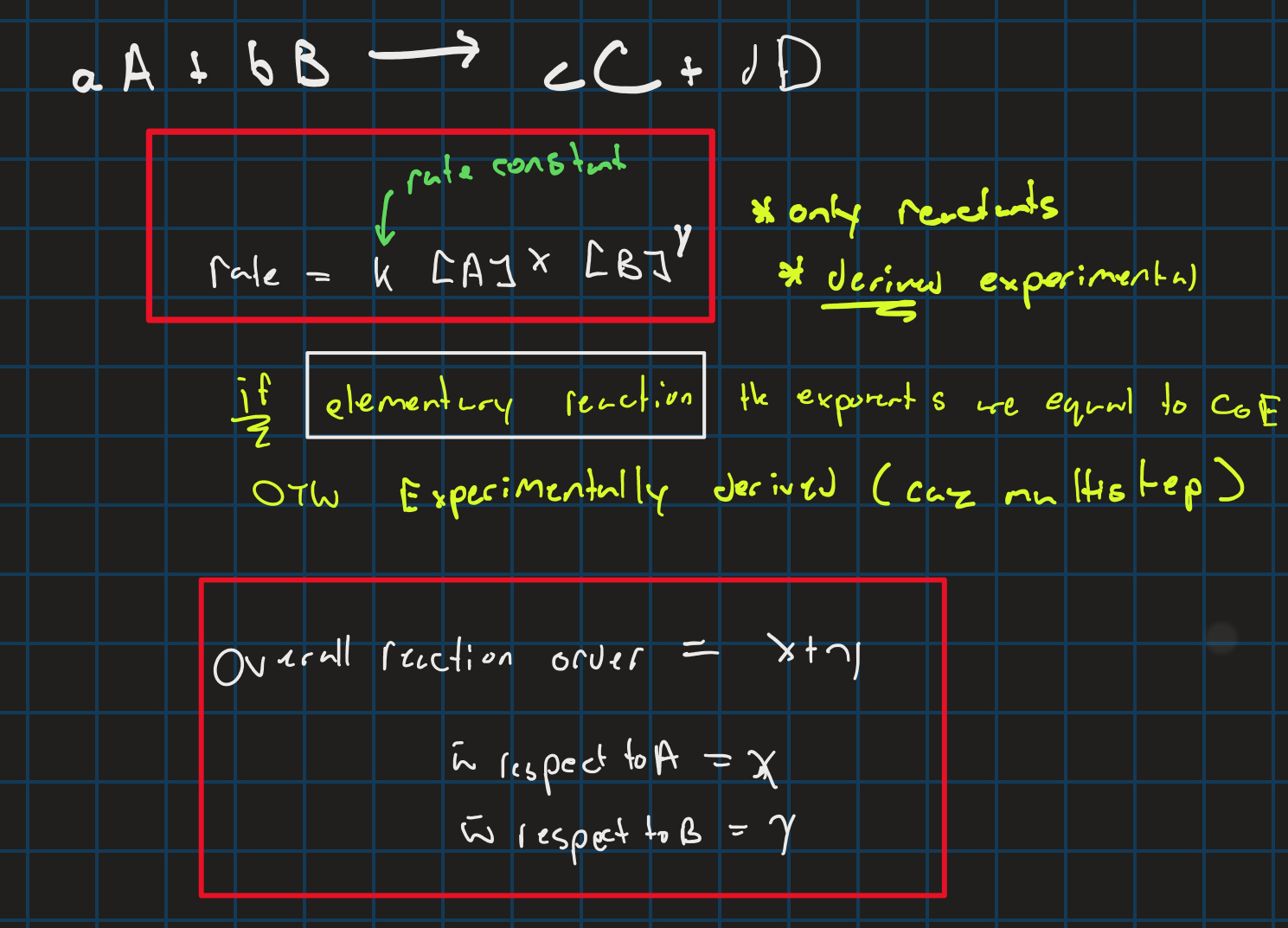
rate law
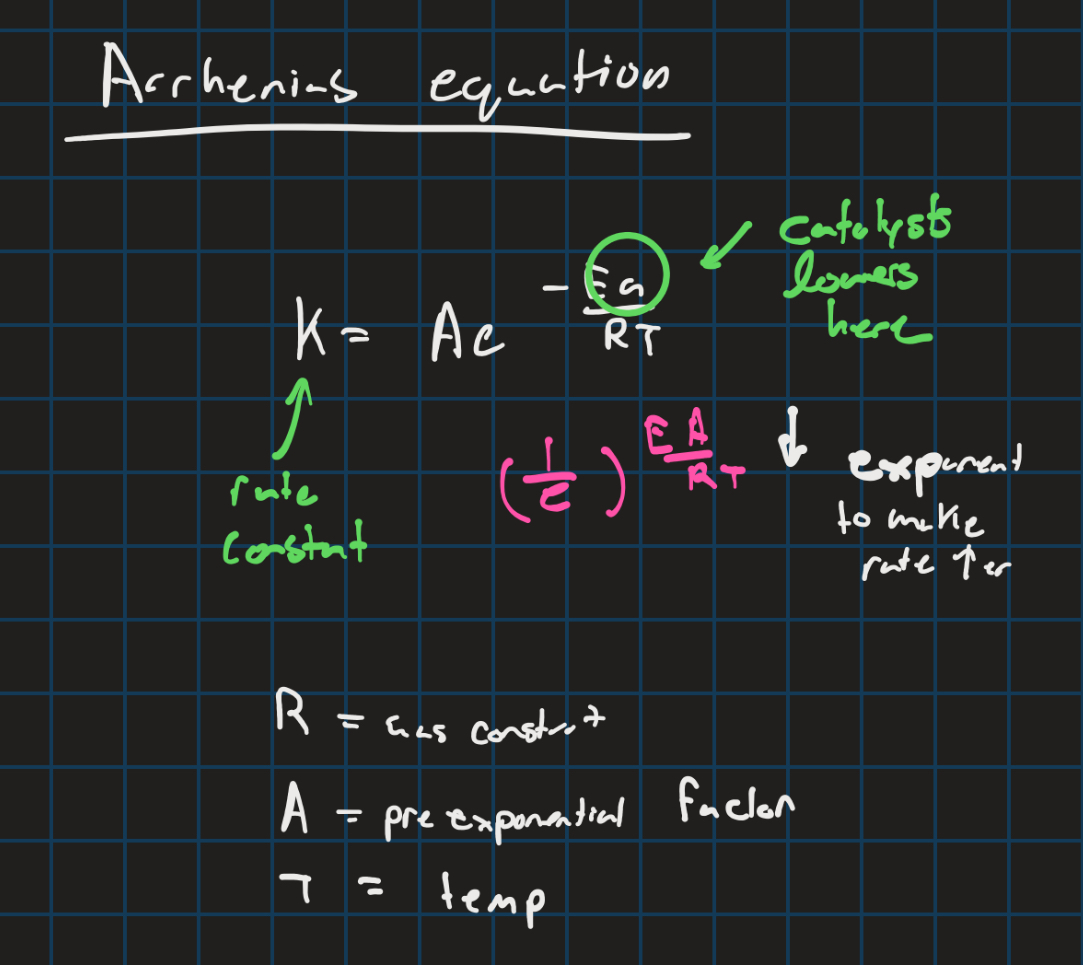
Arrhenius equation
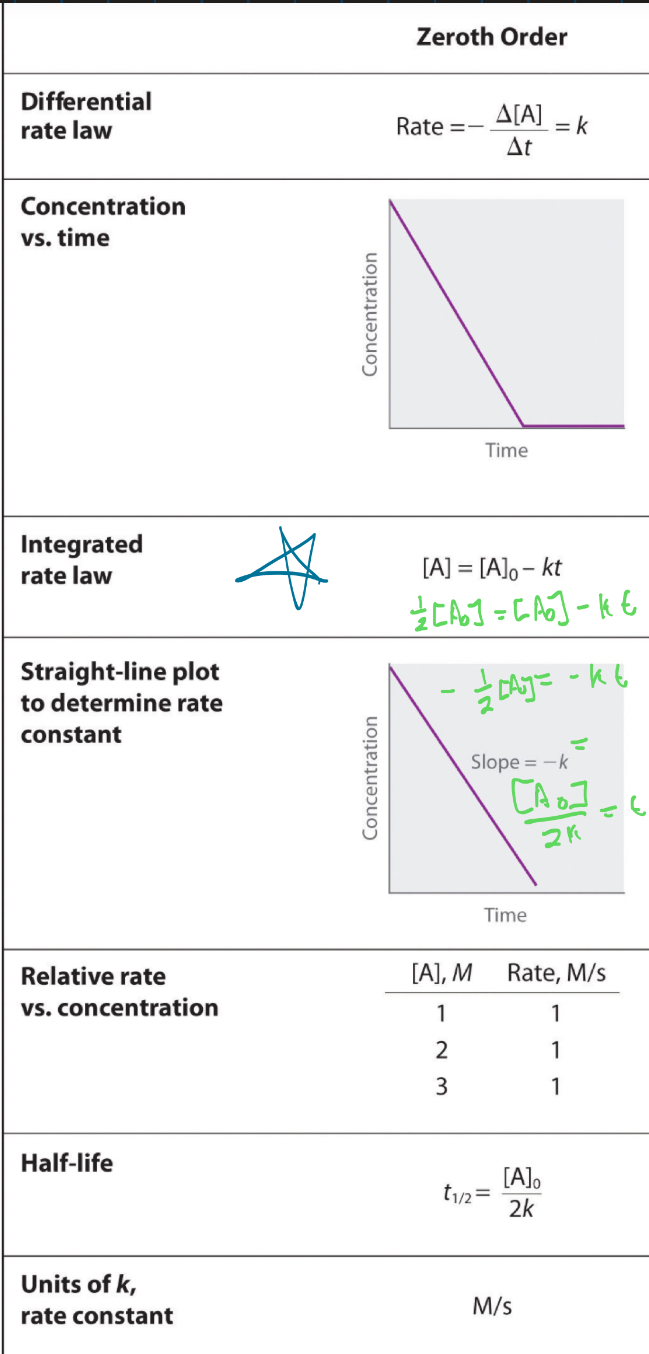
Zeroth order
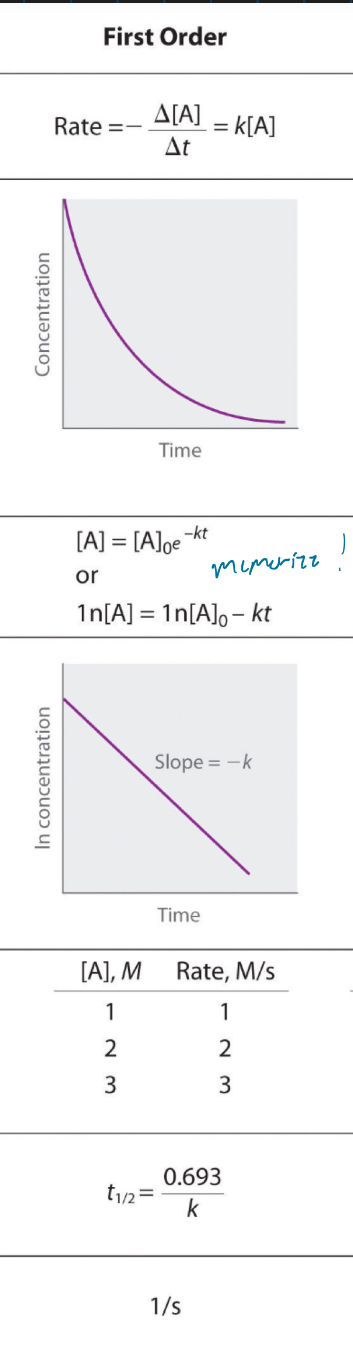
First order
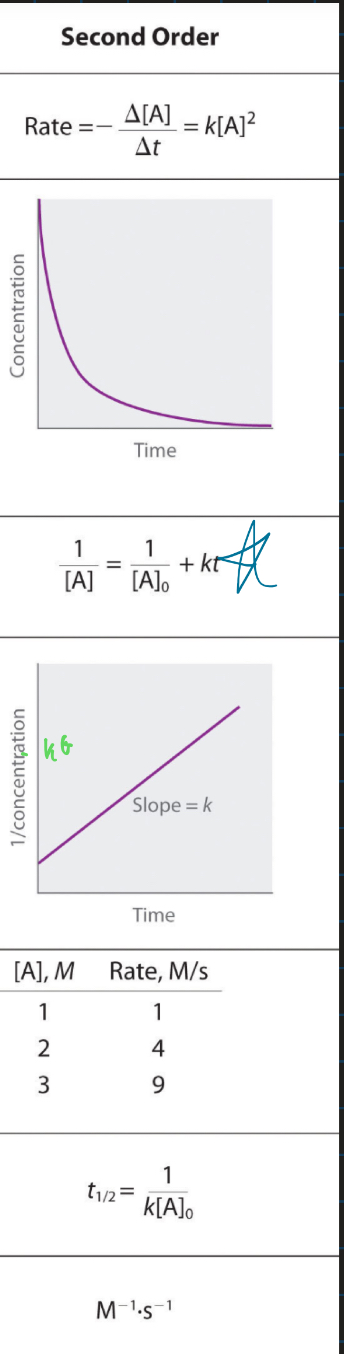
second order
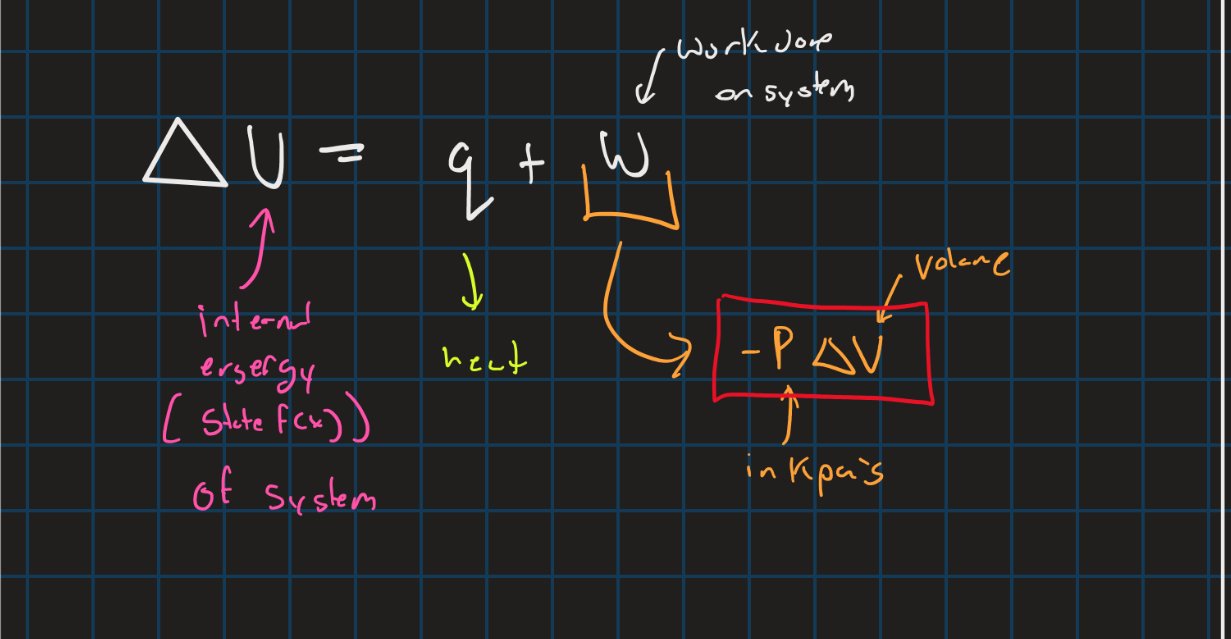
Internal energy of the system
Entropy
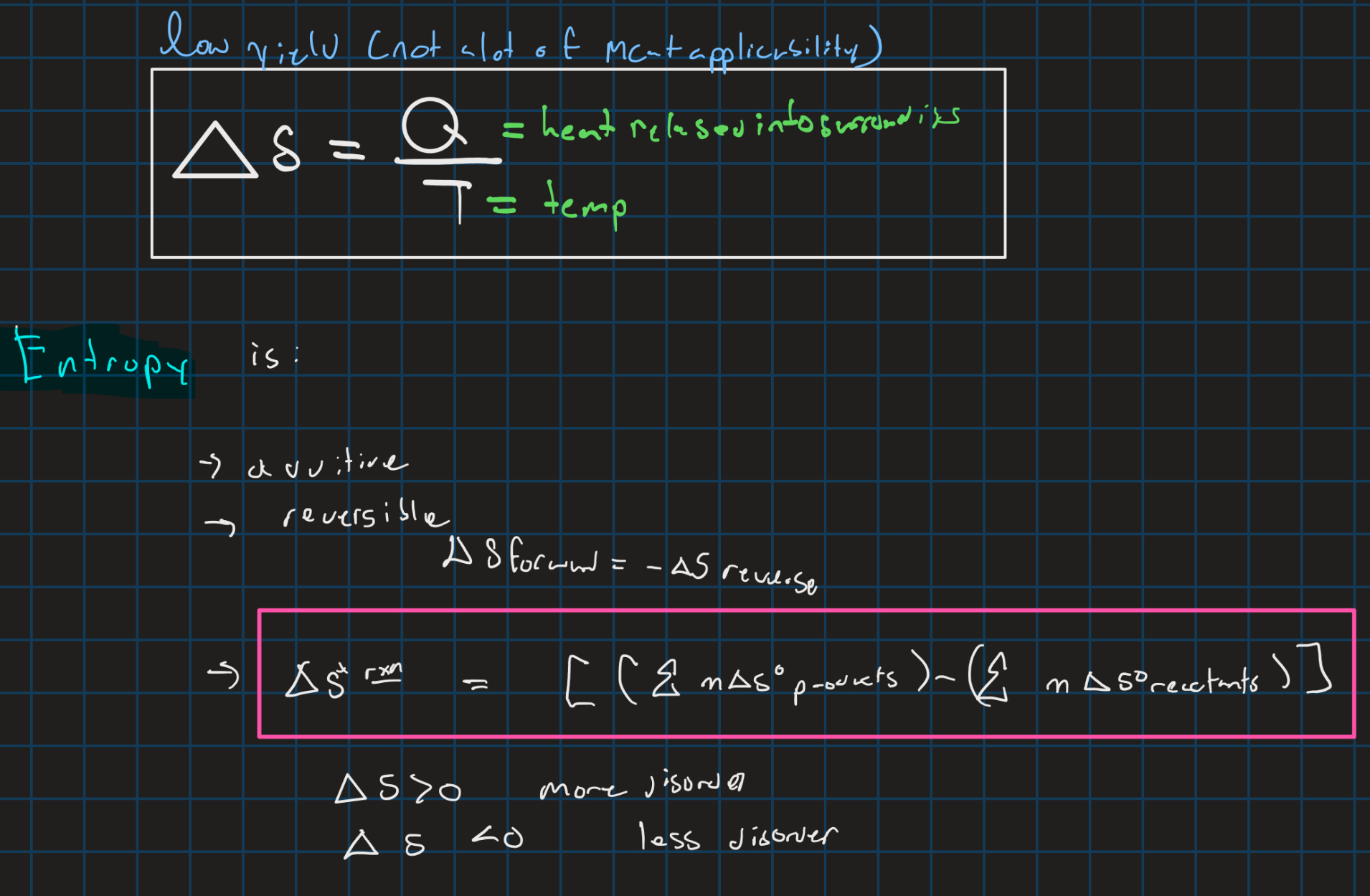
Gibbs free energy
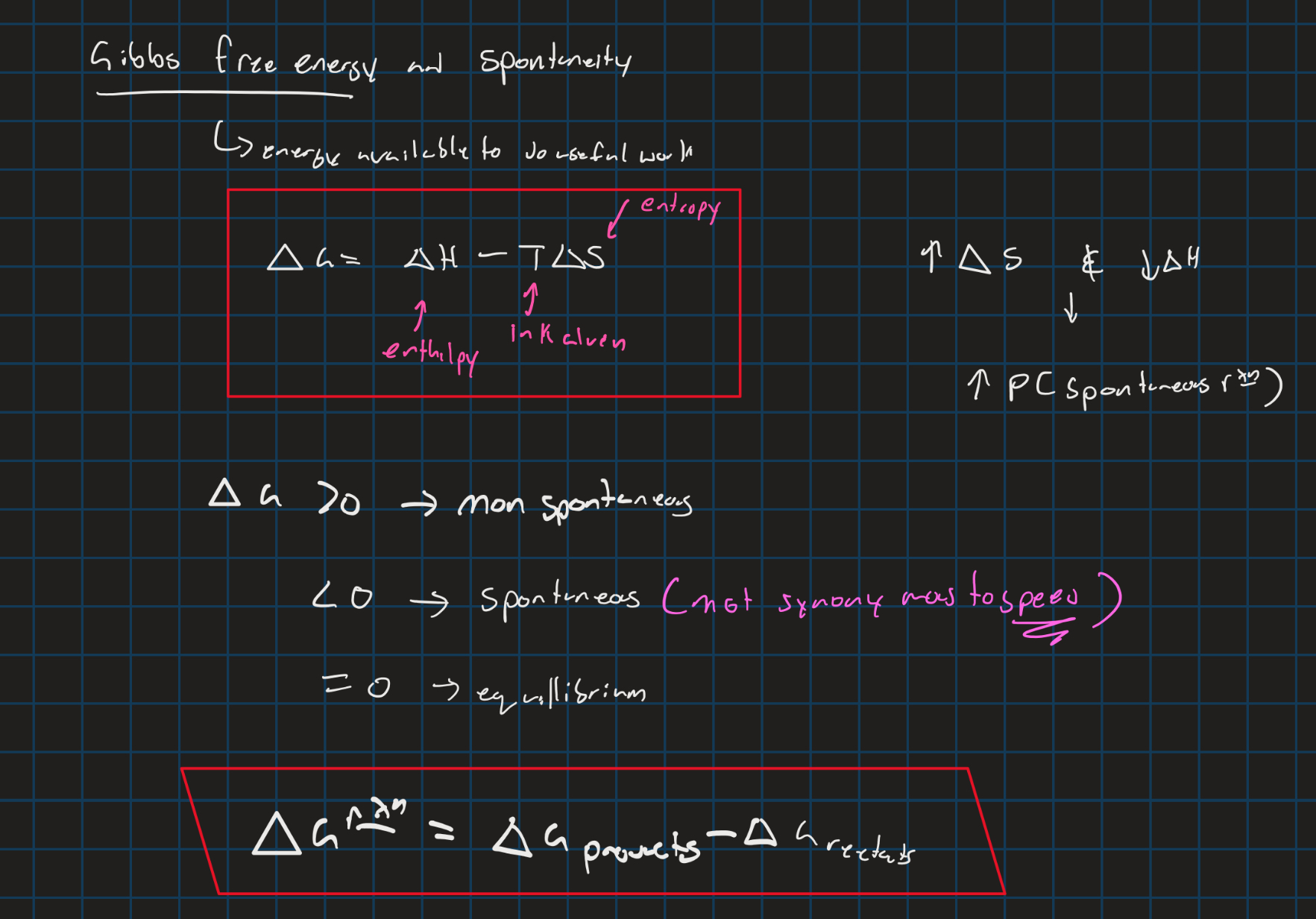
Enthalpy (BDE)
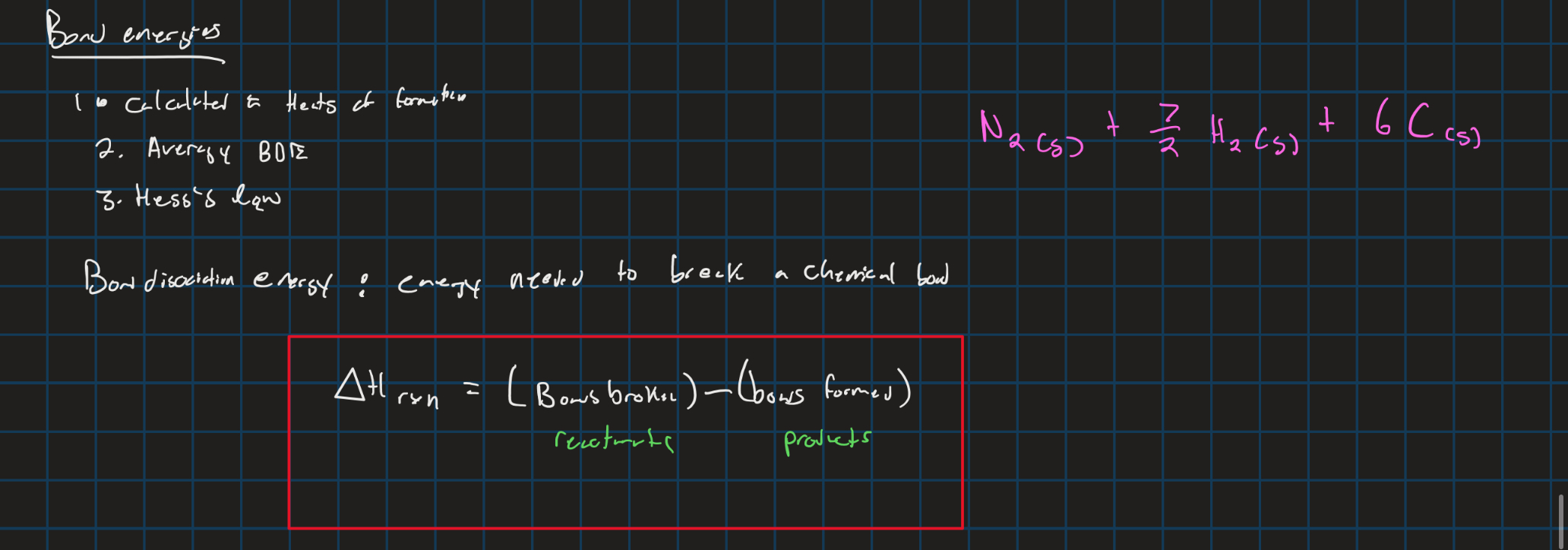
Gibbs free energy with equillibrium constant
Delta G nott
Enthalpy withe heats of formation
products minus reactants
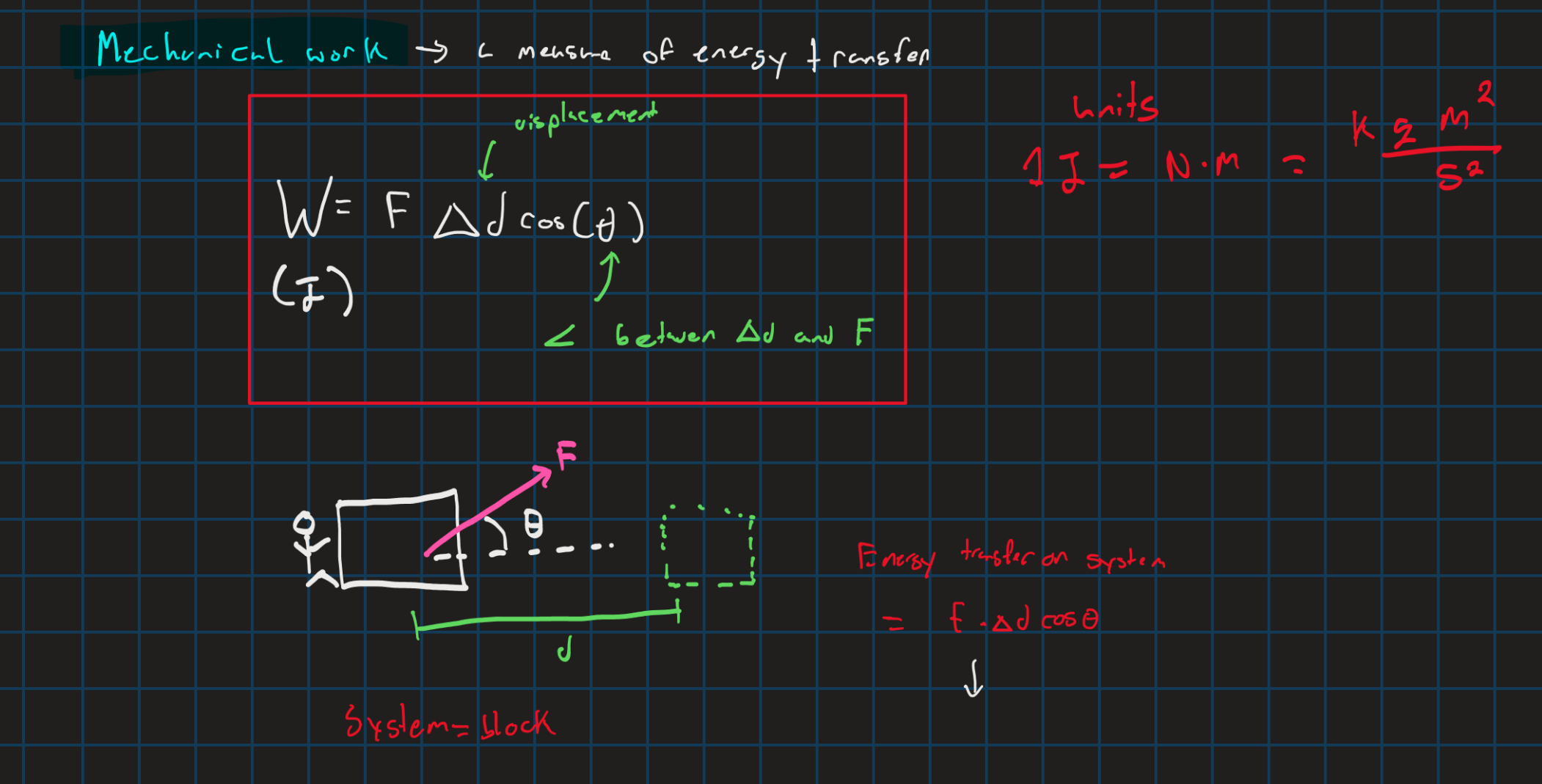
mechanical work
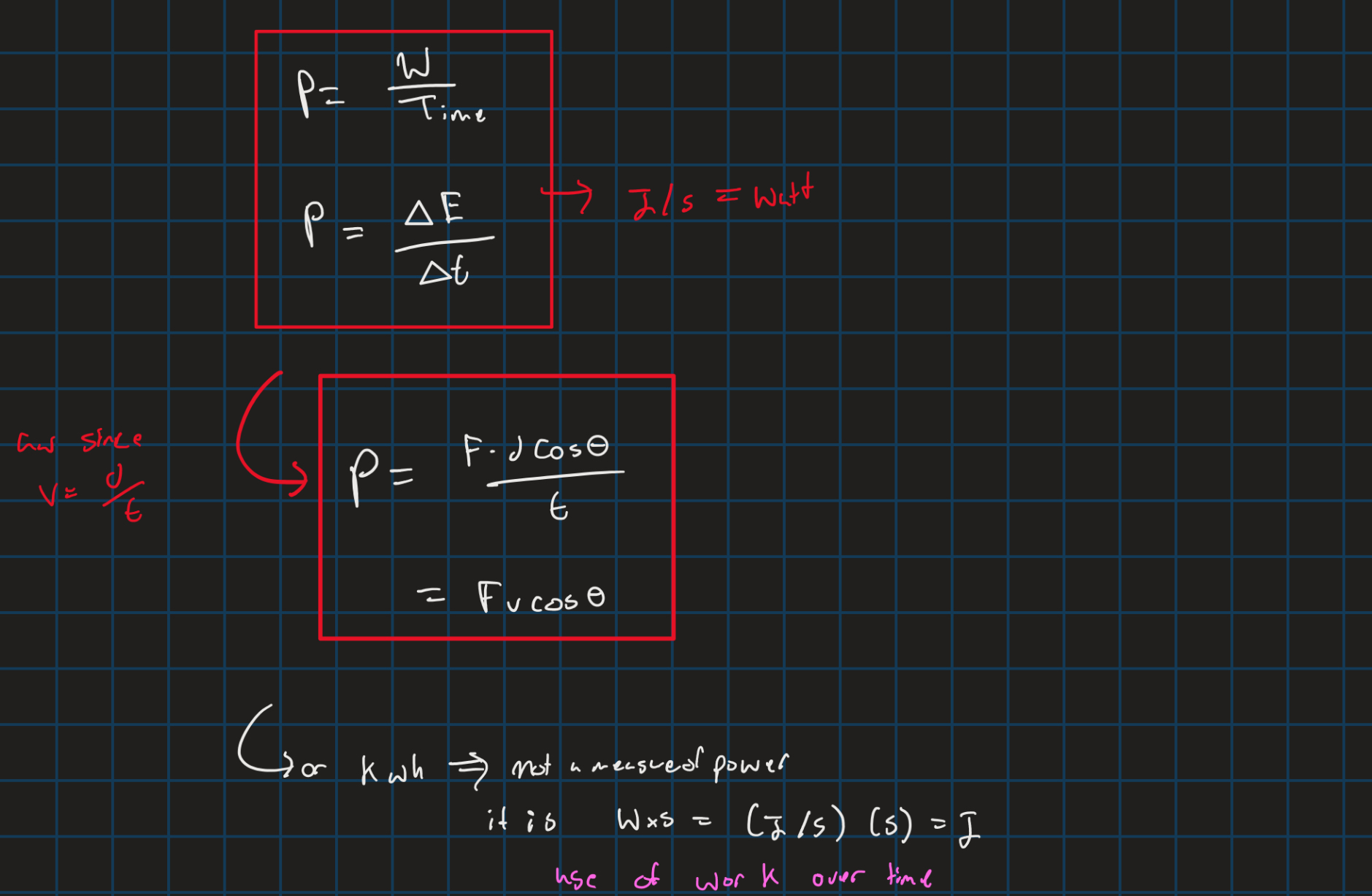
Power
kinetic energy
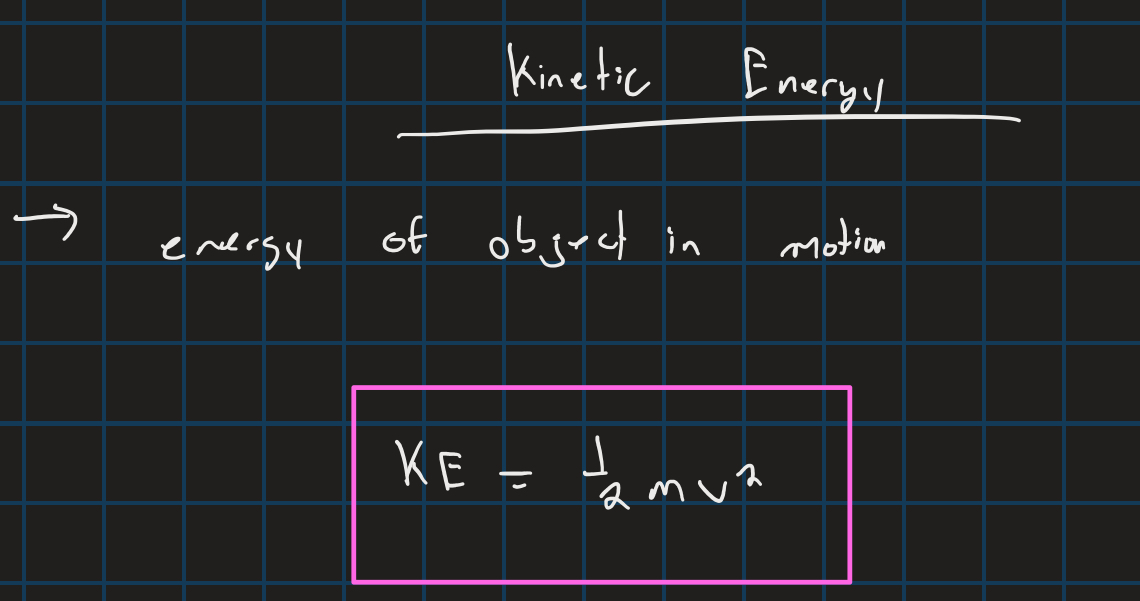
Gravitational potential energy

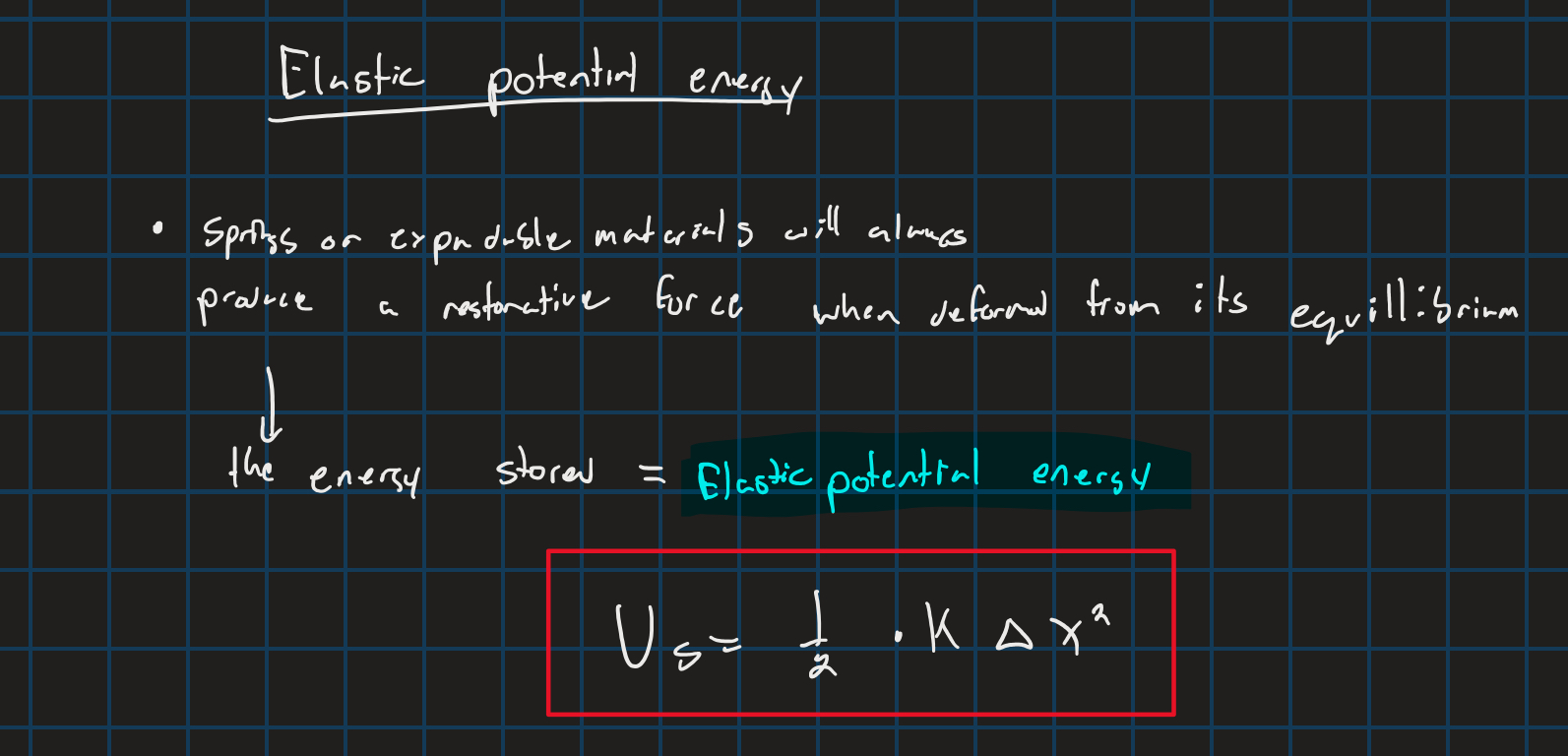
Elastic potential energy
Law of conservation of energy
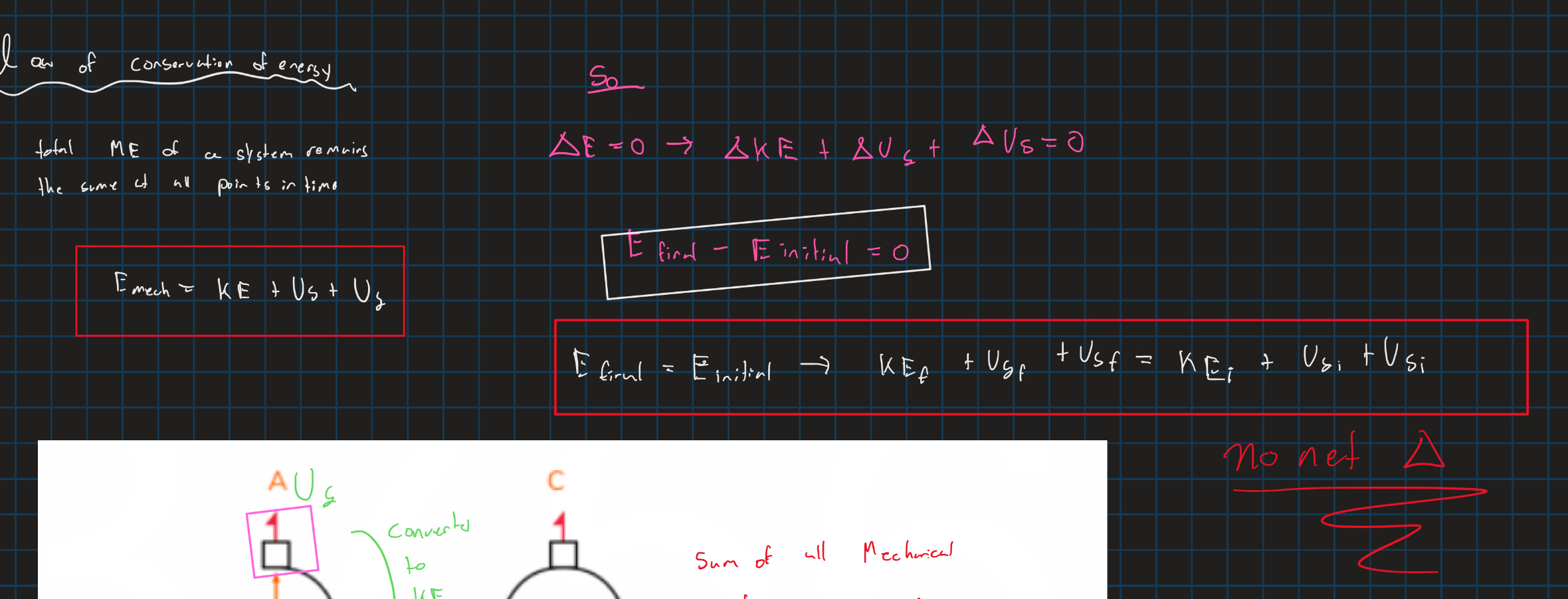
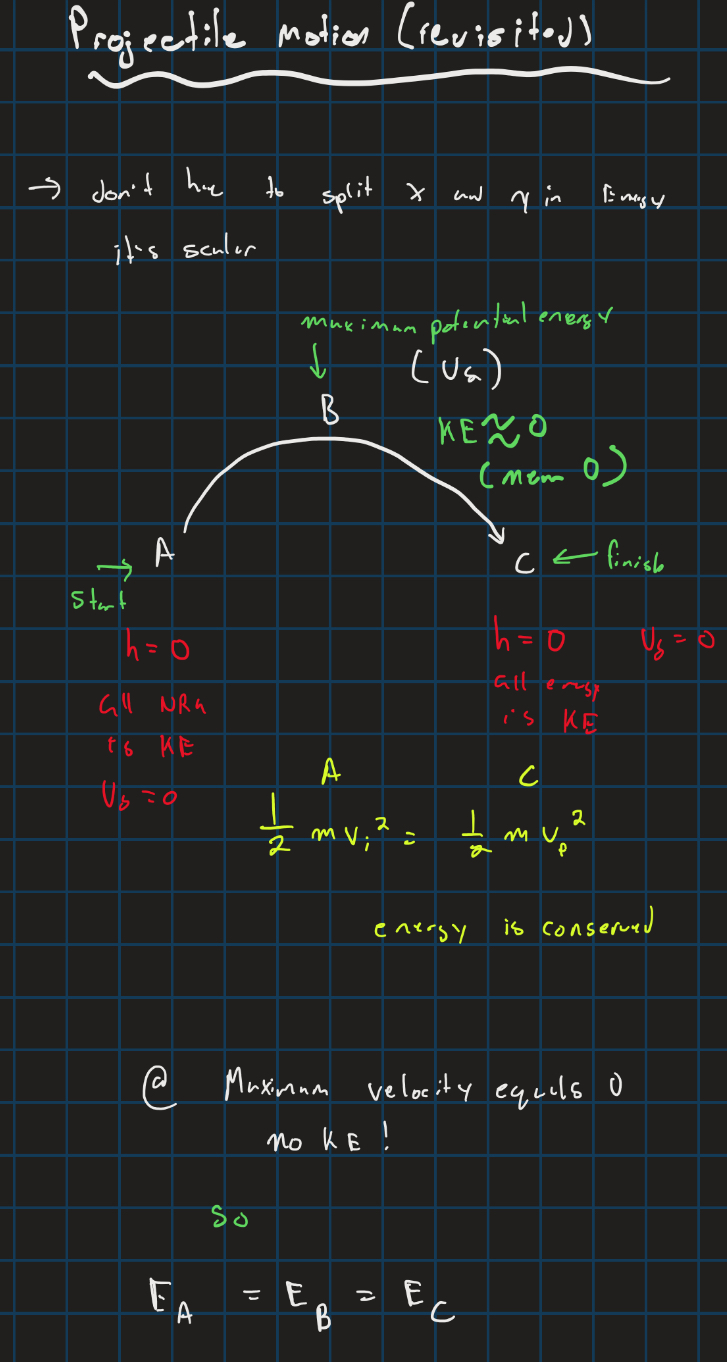
Projectile motion and energy
Work energy and theorem
external work = change in KE + change in Ug + change in Us
