Bio chap 5 - Structure and function of large biological molecules
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
Which of the following statements concerning unsaturated fats is true?
A. They have double bonds in the carbon chains of their fatty acids.
B. They have fewer fatty acid molecules per fat molecule.
C. They generally solidify at room temperature.
D. They are more common in animals than in plants.
E. They contain more hydrogen than do saturated fats having the same number of carbon atoms.
A.They have double bonds in the carbon chains of their fatty acids.
pg 73
The structural level of a protein least affected by a disruption in hydrogen bonding is the_________
Primary level.
primary level of protein folding
order of amino acids determined by inherited genetic information. this is forming the 'backbone' of the protein through its specific sequence of amino acids and side chains present
-connected via peptide bonds ( covalent bonds )
secondary level of protein folding
alpha helix and beta pleated sheets , held together by hydrogen bonds only
-coils and folds with the backbone of the structure built in phase one
tertiary level of protein folding
is the overall shape of a polypeptide resulting from interactions between side chains ( r groups ) of the various amino acids.
-involves hydrophobic interactions ( all the non polar side chains will group up as a cluster at the core of the protein out of contact with water held together by vanderwalls interactions , meanwhile H bonds between the polar sides help stabilize the rest of the tertiary structure.
-covalent bonds are also present through the disulfide bridges formed by the sulfydryl groups further reinforce the shape of the p protein.
( hydrogen bonds, vanderwalls bonds, covalent bonds )
quartenary structure of a protein folding
not all make it here
-must have atlas two or more polypeptide changes required
-collegen is an example of a molecule that makes it to this level
what ultimately determines a proteins structure?
the amino acids in the primary phase
The molecular formula for glucose is C6H12O6. What would be the molecular formula for a polymer made by linking ten glucose molecules together by dehydration reactions?
Remember this linking involved the process of dehydration meaning it expels water for each linkage ( 9 in this case)
10 x C6H12O6 - 9x H2O
=C60H102O51
What structural difference accounts for the functional differences between starch and cellulose?
a. Starch and cellulose differ in the glycosidic linkages between their glucose monomers.
b. Starch is a polymer of glucose, whereas cellulose is a polymer of fructose.
c. Starch can be digested by animal enzymes, whereas cellulose cannot.
a. starch and cellulose differ in the glycosidic linkages between their glucose monomers.
Both starch and cellulose are glucose polymers, but the glycosidic linkages in these two polymers differ.
Glucose can have two slightly different ring structures. When glucose forms a ring, the hydroxyl group attached to the number 1 carbon is positioned either below (alpha) or above (beta) the plane of the ring. In starch, all the glucose monomers are in the alpha configuration.
In cellulose, all the glucose monomers are in the beta configuration. As a result, every other glucose monomer is "upside down" with respect to its neighbors. The differing glycosidic linkages in starch and cellulose give the two molecules distinct three-dimensional shapes, leading to key functional differences.
Which levels of the protein structure may be stabilized by covalent bonds?
Primary, tertiary and quaternary levels of protein structure
The primary structure of a protein is the specific linear sequence of amino acids forming the protein. The amino acids are joined by covalent peptide bonds. Tertiary structure, producing the unique structure of a protein, is stabilized by interactions among the R groups on each amino acid in the protein.
Tertiary structure may be stabilized by covalent bonds, called disulfide bridges, that form between the sulfhydryl groups (SH) of two cysteine monomers. Tertiary structure may also be stabilized by weaker interactions, including hydrogen bonds between polar and/or charged areas, ionic bonds between charged R groups, and hydrophobic interactions and van der Waals interactions among hydrophobic R groups. Many globular proteins are made up of several polypeptide chains called subunits stuck to each other by a variety of attractive forces but rarely by covalent bonds. Protein chemists describe this as quaternary structure.
Do all proteins go through all four levels of protein structuring?
no, Quaternary structure results from the aggregation of two or more polypeptide subunits, and not all proteins are composed of more than one polypeptide.
What is an amino acid ?
def; an organic molecule with both an amino group and a carboxyl group attached by an alpha carbon ( carbon side chain or r group )
the monomer of a polymer 'polypeptide ' many amino acids make up polypeptides through covalent bonds called peptide bonds.
multiple peptide bonds form into a specific molecular shape called proteins
-amino acids can be considered acidic or basic depending on their side chains
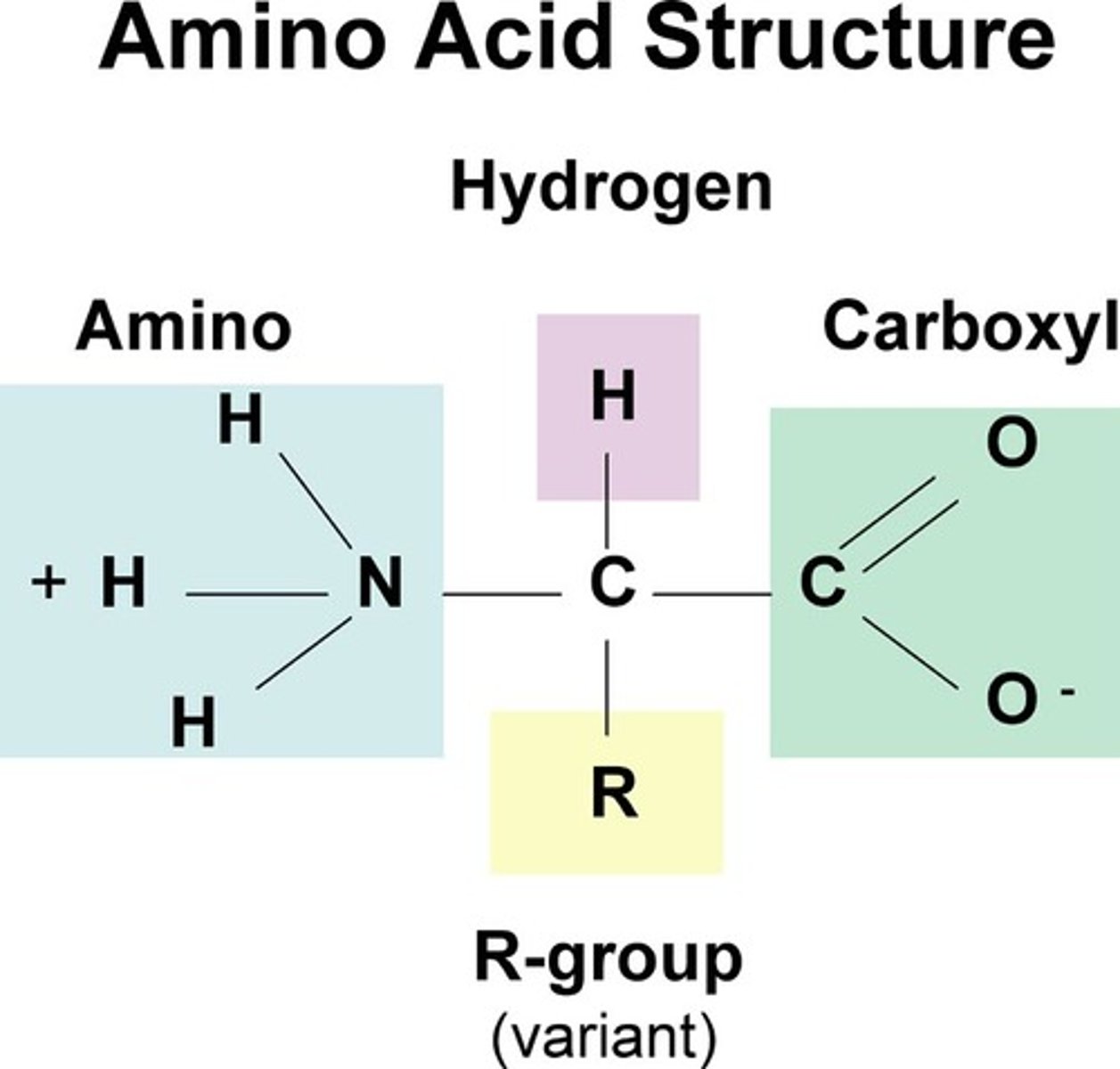
c terminus and n terminus involving polypeptides
side with a single carboxyl end ( c terminus ) side with a single amino ( N terminus )
- the polypeptide chain will end with one each
Which molecule is a nucleotide?
a.The amino acid glycine
b. Deoxyribose
c. ATP
ATP
A nucleotide consists of three parts: a nitrogenous base, a pentose sugar, and one or more phosphate groups. ATP consists of a nitrogenous base (adenine), a pentose sugar, and three phosphate groups.
deoxyribose would be the sugar part and amino acid glycine would be a nitrogenous base
What is a nucleotide?
composed of three parts ;
a five carbon sugar ( pentose ) , a nitrogen containing base , and one or more phosphate groups.
-in a polynucleotide each nucleotide only contains one phosphate group
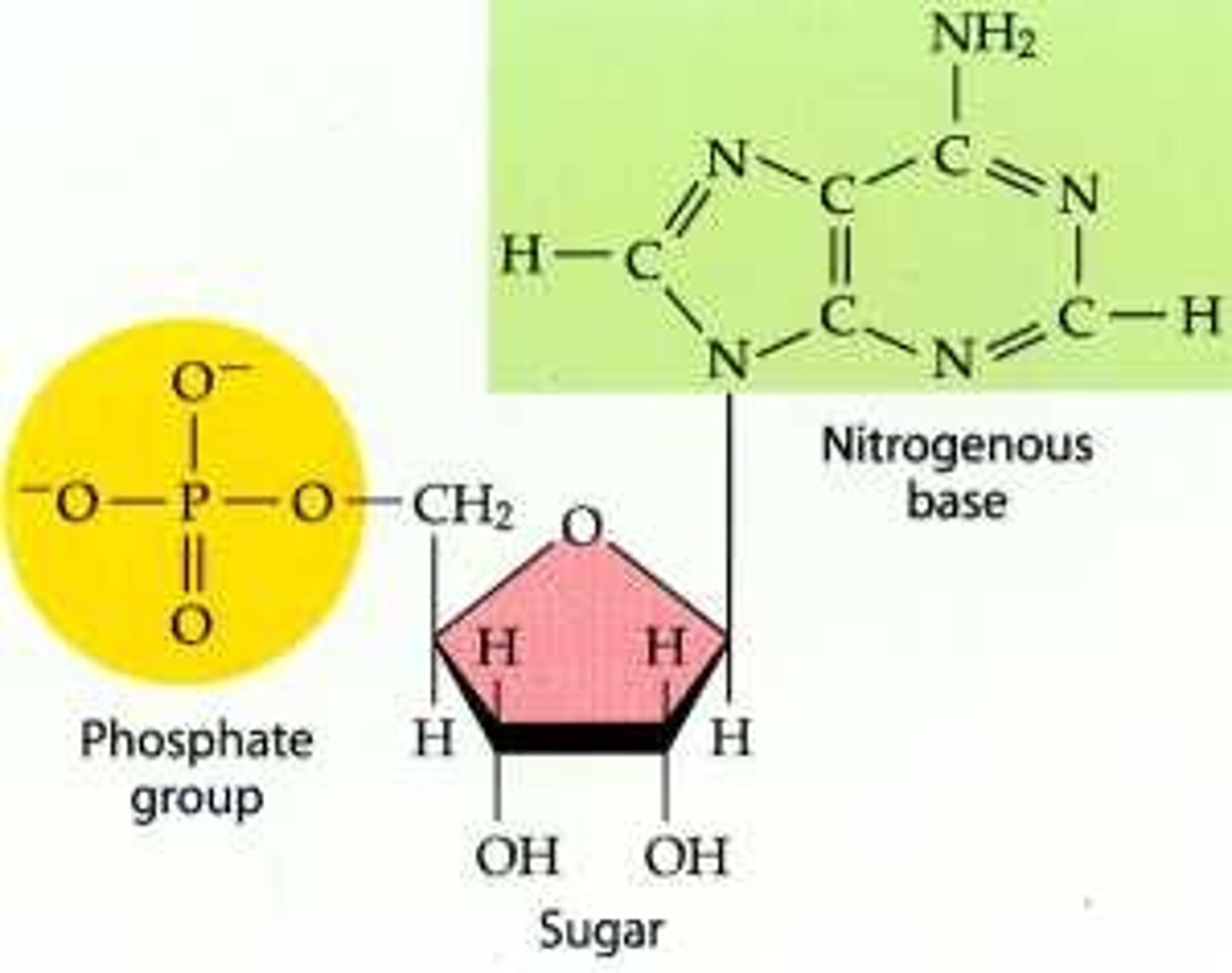
nucleoside
the portion of a nucleotide without any phosphate group is a nucleoside ( nucleobiatch)
what is a nucleic acid?
a polymer made of monomers called nucleotides, it is technically called a polynucleotide as well
- there are two types - DNA and RNA, these enable living organisms to reproduce their couplex components from one generation to the next
-DNA provides its own directions for replication
-DNA also directs RNA sytnetis and through RNA controls protein synthesis through a process called gene expression
nucleic acids - nucleotides - polynucleotides ( nucleic acids)
nucleic acids ( DNA /RNA) make up 1 part of the 3 part nucleotides.
-nucleotides are the monomers of the polymer nucleic acids ( poly nucleotides)
Gene
amino acid sequence of a polypeptide is programmed by a gene. genes consist of DNA which is a nucleic acid
DNA
a type of nucleic acid, the genetic material that organisms inherit from their parents
-each chromosome contains one long DNA molecules
-the DNA does not do all the work rather tells the proteins what to do
RNA
type of nucleic acid, eat gene along a DNA molecule directs synthesis of a type of RNA called messenger RNA
-the m RNAN molecules interacts with the cells protein synthesizing machinery to direct production of a polypeptide which fold into all or part of a protein
-this protein synthesis takes place in a Ribosome
the amino acid sequence of a polypeptide is programed by a discrete unit of inheritance known as a ________
gene
DNA
is a double helix of two polynucleotides referred to as sugar phosphate backbone that run anti parallell ( 5-3)
-these are linked up by hydrogen bonds between the nitrogenous bases which are the inside of the double helix and the the sugar phosphates are on the outside
genomics
- analyzing large sets of genes or analyzing another genome of completely different species to analyze genome
proteonomics
analysis or a large set of proteins, similar to genomics, the translation of proteins sequence can help learn about the DNA sequences that coded them .
pairing of nitrogenous bases DNA / RNA
DNA
- A always pairs with T
- G always pairs with C
RNA
-A always pairs with U , as the T is not present
- G always pairs with C
Which of the following best describes cis-trans isomers?
a. They differ in their spatial arrangement around inflexible double bonds.
b. They are long chains of hydrogen and carbon atoms.
c. They are mirror images of each other.
d. They have the same number of atoms of the same elements but different structures.
e. They differ in the arrangement of covalent bonds and in covalent partners.
A. a. They differ in their spatial arrangement around inflexible double bonds.
tricky thing here is spatial arrangement around inflexible double bonds notes just says 'have the same covalent arrangements but differ in spatial arrangement'
Cis-trans isomers maintain the same covalent partnerships, but the atoms may be arranged differently.
Which of the following statements about the formation of polypeptides from amino acids is true?
A. A bond forms between the carboxyl functional group of one amino acid and the amino functional group of the other amino acid.
B. A bond can form between any carbon and nitrogen atom in the two amino acids being joined.
C. Polypeptides form by condensation or hydrolysis reactions.
D. The reaction occurs through the addition of a water molecule to the amino acids.
A. A bond forms between the carboxyl functional group of one amino acid and the amino functional group of the other amino acid.
Using dehydration; The carboxyl group is one one and and meets up with the amino functional group, use dehydration to extend the peptide bond
The four main categories of large biological molecules present in living systems are _____.
proteins, nucleic acids, carbohydrates, and lipids
Sucrose is formed when glucose is joined to fructose by a(n) _____.
Glycosidic linkages join simple sugars to form polysaccharides.
What is a nucleotide?
a nucleotide in general is composed of three parts: a five carbon sugar , a nitrogen containing ( nitrogenous) base, and one or more phosphate groups.
this protein is crucial to the folding process of of other proteins and will segregate the protein into its own hollow cylinder shielding the protein for proper folding.
chaperonins
all proteins are made of some sort of combination of the ____ amino acids that are available
there are 20 different types of amino acids that make up all proteins
Which stage of protein folding involves the creation of alpha helix structures and beta pleated sheets
the secondary structure
These pleats or helix structures are bonded by hydrogen bonds
Alpha helix
a type of structuring of proteins in the secondary phase of protein structuring. a delicate coil held together by hydrogen bonding between every fourth amino acid.
beta pleated sheets
a type of strutting of proteins developed in the secondary phase of protein structuring.
two or more segments of the polypeptide chain lying side by side called beta strands) are connected by hydrogen bonds between the two parallel segments of the polypeptide backbone.
= these structures can make the core of many globular proteins. ex spider silk, the pleated sheets can give the silk an extra tough quality.
the reactions of the secondary structuring level of a protein are reactions between what parts of the molecules?
polypeptide backbones
In the secondary structure it is important to note that the hydrogen bonds of this secondary level are bonds between the repeating constituents of the polypeptide backbone (not the amino acid side chains.
- within the backbone the oxygen atoms have a partial negative charged, and the hydrogen atoms attached to the nitrogens have a partial positive charge, there fore hydrogen bonds can form between the atoms
the reactions of the tertiary structuring level of a protein are reactions between what parts of the molecules?
interactions between the side chains (R groups)of the various amino acids
Tertiary structure
the overall shape of a polypeptide resulting from interactions between the side chains r groups) of the various amino acids
- this process involves a hydrophobic interaction, as the polypeptide folds into its function shape, amino acids with hydrophobic side chains will cluster at the core of the proteins , keeping out of contact with water, and are held together by van der walls interactions. Meanwhile hydrogen bonds between polar side chains and ionic bonds between positively and negatively charge side chains (R groups ) also help stabilize the tertiary structure.
disulfide bridges
covalent bonds formed in the tertiary structuring, further reinforce the shape of a protein.
They form where two cysteine monomers , which has sulfydryl (-SH)on their side chains are brought close together by folding of the protein. The Sulfers form an S-S bond creating the disulfide bridge
What is an example of a protein that is made in the quartenary structuring of a protein and involves three identical helical polypeptides inter winded into a larger triple helix?
collagen
What is denaturation ?
When a protein for any reason unravels and loses its native shape
- this can happen if physical and chemical conditions of the proteins environment. If the pH, salt concentration temp or other aspects of its environment are altered the weak chemical bonds and interactions of within a protein may be destroyed.
The tertiary structure of a protein includes all of the following interactions except _________ bonds.
-peptide
-ionic
-disulfide bridges
-hydrophobic
-hydrophillic
all are involved with the tertiary structure of a protein except peptide bonds.
Sugars are molecules that have __________ C:H:O and are called __________.
a 1:2:1 ratio ; carbohydrates
The lipids that form the main structural component of cell membranes are __________.
phospholipids
Sugars have a(n) __________ group that interacts with a _________ group that forms ring structures when the dry molecule is placed in water.
carbonyl ( -C=O) , hydroxyl ( -OH)
The type of bond that forms to join monomers (such as sugars and amino acids) into polymers (such as starch and proteins) is a(n) __________ bond.
covalent;
Monomers are joined together by a dehydration reaction in which two molecules are covalently bonded to each other through the loss of a water molecule.
Carbohydrates are used in our bodies mainly for __________.
energy storage and release
While carbs can be use for storage and structuring the primary use for our bodies is energy storage and release.
Glycoside linkage , a covalent bond formed between two ___________ by dehydration reaction is present in the formation of the polymer _______________.
monosaccarides, polysaccarides . Carbohydrates /sugars etc
A polysaccharide that is used for storing energy in human muscle and liver cells is __________.
glycogen
The animal version of this would be starch
The molecule with four fused rings that is found in animal membranes and is the precursor of vertebrate sex hormones is __________.
Cholesterol
cholesterol is grouped under the lipid class as it is a type of steroid.
Steriod
lipids characterized by a carbon skeleton consisting of four fused rings. Different steroids are distinguished by the particular chemical groups attached to this ensemble of rings.
Cholesterol is a steroid and is crucial to molecules in animals which is a precursor from with other steroids, such as the vertebrate sex hormones are synthesized.
One characteristic shared by sucrose, lactose, and maltose is that __________.
they are all disaccharides
All disaccharides or polysaccharides are connected through covalent bonds through dehydration reactions called glycosidic linkages.
A shortage of phosphorus in the soil would make it especially difficult for a plant to manufacture __________.
Nucleic acids are macromolecules that exist as polymers called polynucleotides. DNA (deoxyribonucleic acid) is an example of a nucleic acid. As indicated by the name, each polynucleotide consists of monomers called nucleotides. A nucleotide, in general, is composed of three parts: a nitrogen-containing (nitrogenous) base, a five-carbon sugar (a pentose), and one or more phosphate groups. In a polynucleotide, each monomer has only one phosphate group. The backbone of a nucleic acid consists of alternating sugar and phosphate groups.
Protein molecules are polymers (chains) of __________.
amino acid molecules
The peptide bond is __________.
a covalent bond joining amino acids together to form a polypeptide
Generally, animals cannot digest (hydrolyze) the glycosidic linkages between the glucose molecules in cellulose. How then do cows get enough nutrients from eating grass?
microorganisms in their digestive tracts hydrolyze the cellulose to individual glucose units.
The polysaccharide called cellulose is a major component of the tough walls that enclose plant cells. Like starch, cellulose is a polymer of glucose, but the glycosidic linkages in these two polymers differ. In starch, all the glucose monomers are in the α configuration. In contrast, the glucose monomers of cellulose are all in the β configuration, making every glucose monomer "upside down" with respect to its neighbors. Enzymes that digest starch by hydrolyzing its α linkages are unable to hydrolyze the β linkages of cellulose because of the distinctly different shapes of these two molecules. In fact, few organisms possess enzymes that can digest cellulose. Cows have digestive chambers populated by microorganisms that can produce certain hydrolytic enzymes that cows cannot. The enzymes hydrolyze (digest) the cellulose polymer into glucose monomers.
When comparing saturated and naturally occurring unsaturated fats, the unsaturated fats have __________ and are __________ at room temperature.
cis double bonds; liquids
The terms saturated fats and unsaturated fats are commonly used in the context of nutrition. These terms refer to the structure of the hydrocarbon chains of the fatty acids. If there are no double bonds between carbon atoms composing a chain, then as many hydrogen atoms as possible are bonded to the carbon skeleton. Such a structure is said to be saturated with hydrogen, and the resulting fatty acid is therefore called a saturated fatty acid.
An unsaturated fatty acid has one or more double bonds, with one fewer hydrogen atom on each double-bonded carbon. Nearly all double bonds in naturally occurring fatty acids are cis double bonds, which cause a kink in the hydrocarbon chain wherever they occur.
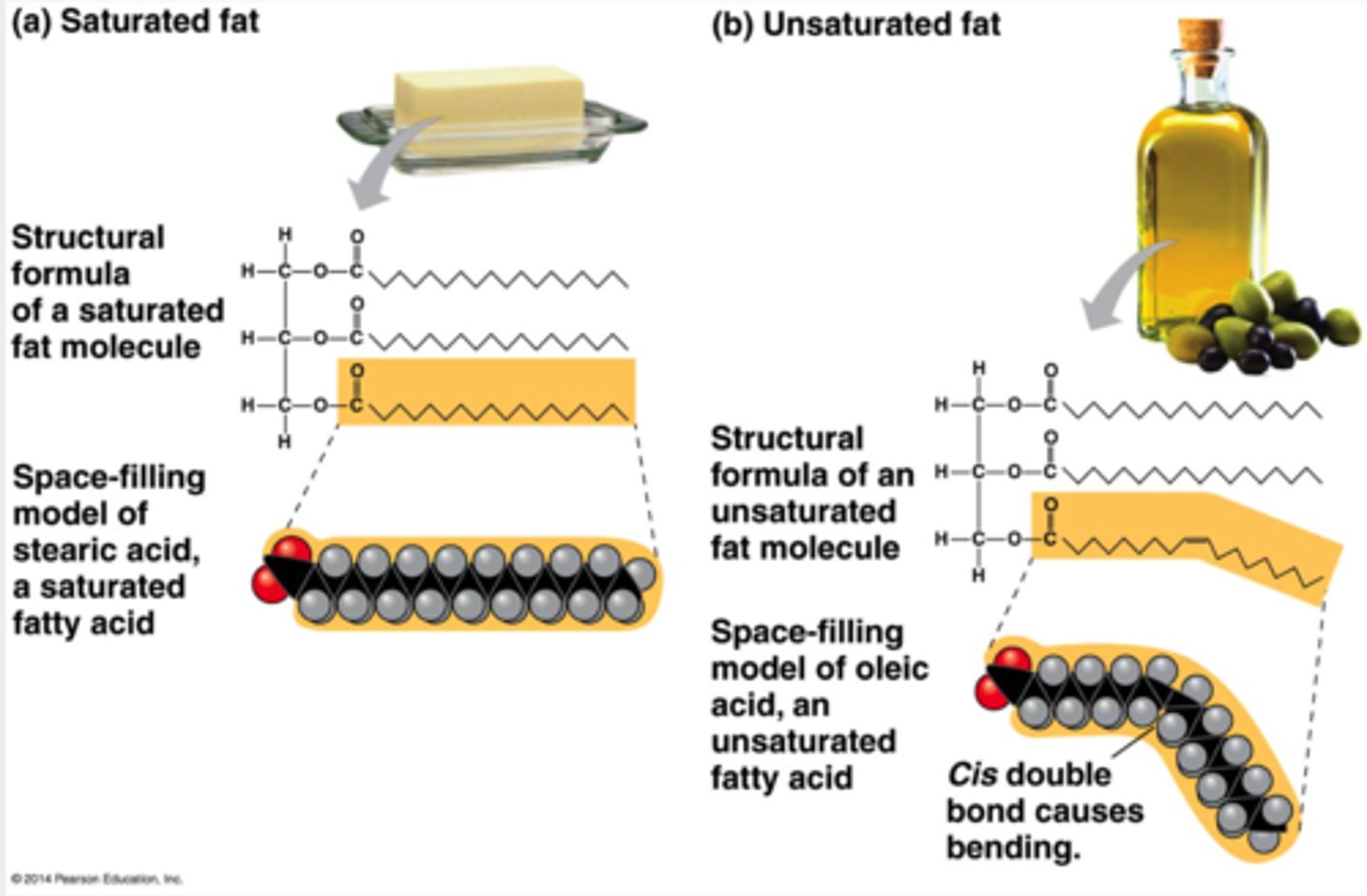
saturated fats lack this of which unsaturated fats possess.
double bonds, and their flexibility allows the fat molecules to pack together tightly
What is a fat?
not polymers,
large molecules assembled from smaller molecules by dehydration reactions.
-contracted from two kinds of smaller molecules; Glycerol and fatty acids
-Glycerol is an alcohol in which each of its three carbons bears a hydroxyl group.
-the rest of the skeleton consists of a hydrocarbon chain. (hydrophobic)
-in making a fat three fatty acid molecules are joined to glycerol by an ester linkage , a bond formed by a dehydration reaction between hydroxyl and carboxyl . resulting fat is also called a triacyglycerol
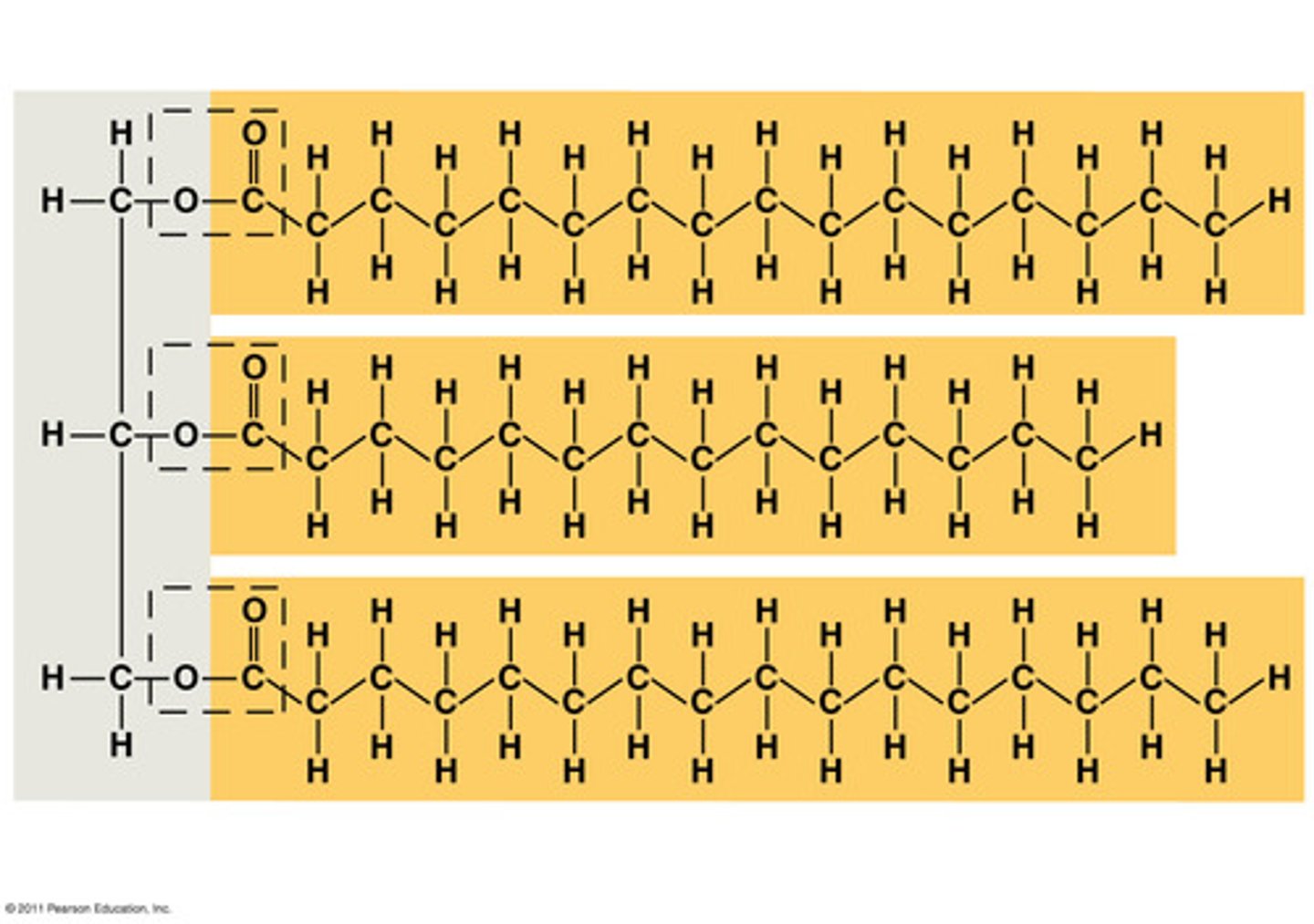
Ester linkage
the linkage that takes places in fats
a bond formed by a dehydration reaction between hydroxyl and carboxyl . resulting fat is also called a triacyglycerol
What is a fatty acid?
has a long carbon skeleton , usually 16-18 carbons in length. The carbon t one en doc the skeleton is part of a carboxyl group, the functional group that gives these molecules the name fatty acid.
The components of nucleic acids are __________.
a nitrogenous base, a pentose sugar, and a phosphate
in which these combined from a nucleotide
The sex hormones estrogen, progesterone, and testosterone belong to which class of molecules?
lipids
Nitrogenous bases are classified as either purines or pyrimidines. Examples of purines are __________
adenine and guanine
To build a nucleotide, let's first consider the nitrogenous bases. Each nitrogenous base has one or two rings that include nitrogen atoms. (They are called nitrogenous bases because the nitrogen atoms tend to take up H++ from solution, thus acting as bases.) There are two families of nitrogenous bases: pyrimidines and purines. A pyrimidine has one six-membered ring of carbon and nitrogen atoms. The members of the pyrimidine family are cytosine (C), thymine (T), and uracil (U). Purines are larger, with a six-membered ring fused to a five-membered ring. The purines are adenine (A) and guanine (G). The specific pyrimidines and purines differ in the chemical groups attached to the rings. Adenine, guanine, and cytosine are found in both DNA and RNA; thymine is found only in DNA and uracil only in RNA.
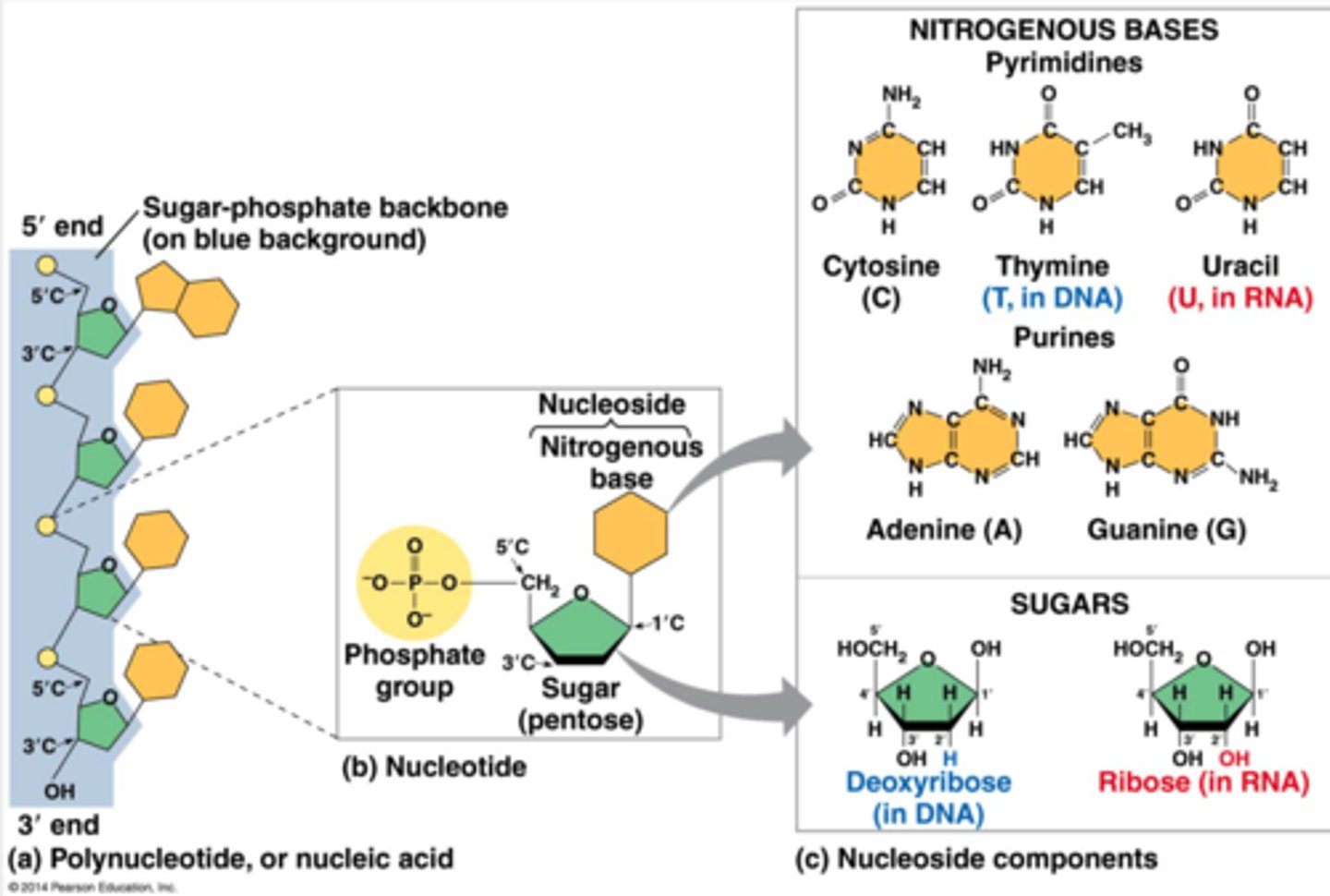
purine
a nitrogenous base
larger six membered ring fused to a five membered ring.
A (adenine) and G (guanine).
nitrogenous bases
two types purines and pyrimidine.
-part of a nucleotide, that has one or two rings that include nitrogen atoms. they are are called nitrogenous bases because the nitrogen atoms tend to take up H+ from solutions thus acting as bases.
ATGC (adenine , thymine, guanine, cytosine, uracil)
the specific pyrimidines and purines differ in the chemical groups attached to the rings. ACG are found both in DNA and RNA while T is found only in DNA and U is only in RNA
nucleoside
the portion of a nucleotide without any phosphate group
- so it has a five carbon sugar * pentose ) and a nitrogenous base but missing the phosphate.
pyrimidine
a nitrogenous base , has one six membered ring of carbon and nitrogen atoms.
-members of the pyrimidine family are cytosine (C) thymine (T) and uracil (U)
Which of the following is a polymer?
Fructose, a component of sucrose
cellulose, a plant cell wall component
glucose, an energy rich molecule
testosterone, a steroid hormone
tryglycerol, or a fat
cellulose.
The macromolecules in three of the four classes of life's organic compounds—carbohydrates, proteins, and nucleic acids—are chain-like molecules called polymers. A polymer is a long molecule consisting of many similar or identical building blocks linked by covalent bonds, much as a train consists of a chain of cars. The repeating units that serve as the building blocks of a polymer are smaller molecules called monomers. The polysaccharide cellulose is a major component of plant cell walls. It is a polymer composed of many glucose monomers joined together by glycosidic linkages
fructose is a monomer of sucrose
The secondary structure of a peptide backbone is stabilized by __________ forming either a(n) __________ or a(n) __________.
hydrogen bonds; alpha helix ; beta pleated
describe a nucleotide
To build a nucleotide, let's first consider the nitrogenous bases. Each nitrogenous base has one or two rings that include nitrogen atoms. (They are called nitrogenous bases because the nitrogen atoms tend to take up H++ from solution, thus acting as bases.) There are two families of nitrogenous bases: pyrimidines and purines. A pyrimidine has one six-membered ring of carbon and nitrogen atoms. The members of the pyrimidine family are cytosine (C), thymine (T), and uracil (U). Purines are larger, with a six-membered ring fused to a five-membered ring. The purines are adenine (A) and guanine (G). The specific pyrimidines and purines differ in the chemical groups attached to the rings. Adenine, guanine, and cytosine are found in both DNA and RNA; thymine is found only in DNA and uracil only in RNA.
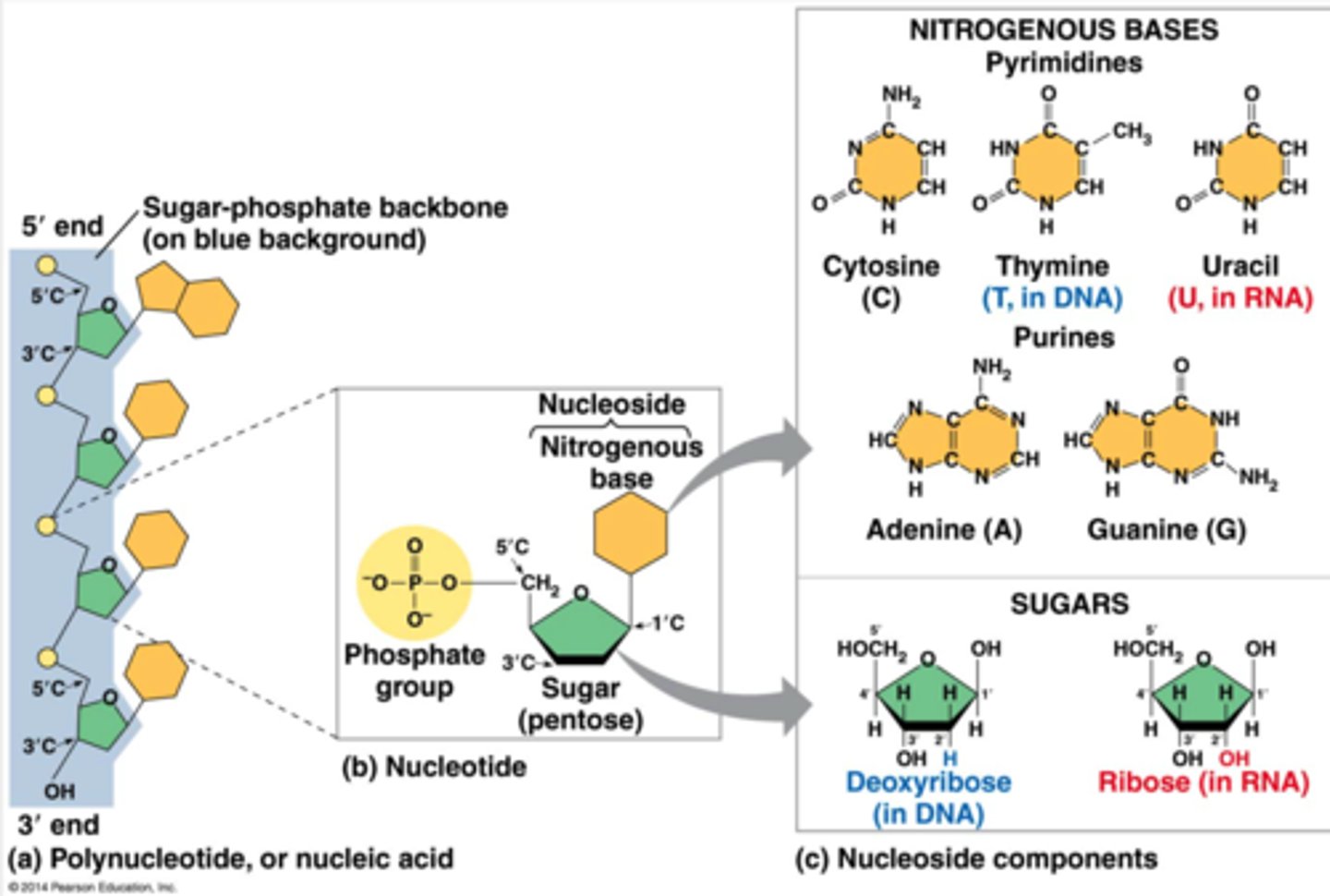
the most common monosaccaride
glucose
What are some criteria for classifying sugars?
through their glycosidic linkages an positioning of the hydroxyl and carbonyl groups , keynote or aldehyde
What major form does a starch molecule take?
amylose and amylopectin ( straight, branched)
What is the most abundant organic compound on Earth?
cellulose , plants , duh.
This is a three carbon alcohol found in most fats.
glycerol - together with three fatty acid chains ( chains of long carbon skeleton with a carboxyl group at the end )
together form a tricylglycerol
These are lipids characterized by a carbon skeleton consisting of four fused rings
steroids , cholesterol is a type of steroid. steroid is a type of lipid,
cholesterol is important and decided the human sex cell
_________ account for more than 50% of the mass of most cells
proteins
most enzymes are these __________
proteins