Thermal properties of matter
1/17
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
To what temperature can Mercury be used to calculate heat?
356 degrees Celsius
What has the highest specific heat capacity
Water
How many joules are present in 1 calorie
4.186 joules
What is the coefficient of specific heat capacity for water
1 calorie/gm
What is the value of the coefficient of specific heat capacity for ice
½ calorie/gm
What is the coefficient of specific heat capacity for steam
0 calorie/gm
del
What is the value of latent heat for ice → water (or) water → ice (in cal/g)
80 cal
What is the value of latent heat for water → steam (or) steam → water (in cal/g)
540 cal
What is the formula for thermal resistance, and also explain its constituent parts
R = L/A*K (Where L → length of conductor ; A → cross sectional area ; k → thermal conductivity→ measure of how well a substance conducts heat)
What is the definition and formula for heat current; Why do we use it
The amount of heat passed per unit length. We use this to find out the rate of heat transfer in a body that is experiencing heat energy
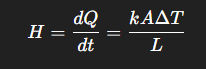
What is the formula for resistance in parallel
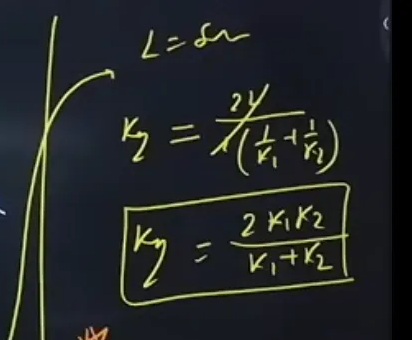
What is Emissive power and what is its formula
It is the amount of heat energy that is being emitted from the body per unit area per unit second
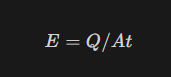
What is the formula for emissive power if a body is at a temperature T
1. Tell its relation with the formula
What is the name of the constant that is being used
E = σ T⁴
E ∝ T⁴
Stephens constant
What is the value of Wien’s Displacement Constant
2.9×10-3 metre Kelvin
What does Wein’s law tell us?
Tells you the wavelength at which the blackbody emits maximum energy per unit wavelength — i.e., the peak of its radiation curve.
What is the formula for Newton’s cooling
Here,
T → temperature
t → time
To → Surrounding temperature
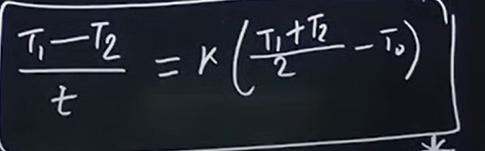
What is the solar constant
The amount of energy released by the sun, which is then absorbed at a distance r by a body, per unit area of the absorbed body that is a planet