BCMB2901 Metabolism
1/272
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
273 Terms
L01
Introduction to Metabolism
When we lose weight, where does the fat go.
a. Lost as energy/heat
b. Carbon dioxide + water
c. Becomes muscle
d. Faeces
e. Don't know
b. Carbon dioxide + water
Which of the following everyday actions could alter
your metabolic efficiency?
a. Eating a high-sugar snack
b. Skipping breakfast
c. Exercising for 30 minutes
d.All of the above
d.All of the above
Explain the catabolic and anabolic pathway
Catabolic pathways
1. Break down larger molecules into smaller ones
2. General strategy to extract H/e- to be delivered to the electron transport chain
3. Sometimes to make ATP directly (substrate level phosphorylation)
Anabolic pathways
1. Build larger molecules from smaller substrates
2. Generally requires input of energy (ATP) or reducing power (NADPH, NADH).
Energy is required to do work.
Name three types of work and their examples.
Chemical work, e.g. building molecules.
Transport work, e.g. pushing concentrations uphill.
Mechanical work, e.g. breathing, contracting muscles, hearts pumping.
The relative rates of catabolic (ATP-generating pathways) and anabolic (ATP-utilising pathways) are regulated by the energy state within the cell. Explain how cell calculates the energy charge and why [AMP] is the most important.
Energy charge = ([ATP] + 1/2[ADP]) / ([ATP] + [ADP] + [AMP]). [AMP] is most important because [AMP] is only in the denominator, anything that is going to make a big change the numerical output of the equation is a change in [AMP].
When energy charge is high, we do what?
When energy charge is low, we do what?
When energy charge is high, we can build some fats for later use.
When energy charge is low, we break down fats.
What is the role of kinases?
What is a phosphorylation reaction?
Kinases catalyse a phosphorylation reaction.
A phosphorylation reaction is a biochemical process in which a phosphate group (PO4³-) is added to a molecule, typically a protein, lipid, or sugar.
What is the role of phosphatases?
What is a dephosphorylation reaction?
Phosphatases catalyse a dephosphorylation reaction.
A dephosphorylation reaction is the biochemical process in which a phosphate group (PO₄³⁻) is removed from a molecule, typically a protein, lipid, or nucleotide.
What is the role of phosphorylases?
What is a phosphorolysis reaction?
Phosphorylases catalyse a phosphorolysis reaction.
A phosphorolysis reaction uses inorganic phosphate to break things apart.
What is the role of synthases?
Synthases catalyse condensation reactions in which no nucleotide triphosphate is required.
What is the role of synthetases?
Synthetases catalyse condensation reactions that require a nucleotide triphosphate.
What do Nucleotide triphosphates include?
Nucleotide triphosphates (NTPs) include ATP (adenosine triphosphate) and GTP (guanosine triphosphate), along with other triphosphates like CTP (cytidine triphosphate), TTP (thymidine triphosphate), and UTP (uridine triphosphate).
What are some features of Dehydrogenases?
Dehydrogenases catalyse oxidation-reduction reactions
Dehydrogenases usually use NAD+/FAD as cofactors
Dehydrogenases are named for the substrate that is oxidised by NAD+/FAD
For example: pyruvate + NADH ⇌ lactate + NAD+ (NADH is oxidised to NAD as it loses electrons, and pyruvate is reduced to lactate as it gains electrons)
Reactions that involve NAD, FAD, NADH, FADH2 are generally involving what?
Dehydrogenases
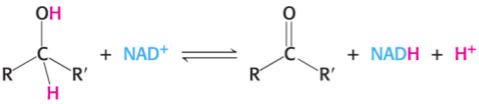
What is the full name of NAD+
NAD+ likes to oxidise what group and turn the group and itself into what?
Nicotinamide adenine dinucleotide
NAD+ likes to oxidise the C—OH group (—CH2—CHOH—) and turn the group into C=O and itself into NADH.
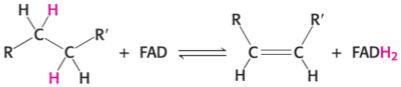
What is the full name of FAD
FAD likes to oxidise what group and turn the group and itself into what?
Flavin adenine dinucleotide
FAD likes to oxidise the C—H single bond (—CH2—CH2—) and turn it into —CH=CH— and turn itself into FADH2
What are three parts of a CoA?
Adenosine 3′,5′-diphosphate
Pantothenic acid
β-Mercapto-ethylamine
Coenzyme A is the carrier of what group?
Coenzyme A is great for trapping what within the cell?
Coenzyme A is the carrier of acyl groups (including acetate or fatty acyl).
Coenzyme A is great for trapping metabolites within the cell.
What are three stages of metabolism?
Stage 1: Glycolysis for glucose, β-oxidation for fatty acids, or amino acid catabolism.
Stage 2: Citric Acid Cycle (or Kreb cycle)
Stage 3: Electron Transport Chain and ATP synthesis
What are the seven big concepts of metabolism? What is the implication?
1. The H/e- carriers are in short supply
2. ADP is in short supply
3. ATP is really stable
4. The inner mitochondrial membrane is impermeable to protons
5. Protons only flow into the matrix if the ATP is being made
6. The proton pumps don’t work if the proton gradient is very high
7. No proton pumping, no H/e- movement down the ET chain
Implication of the seven big concepts: if I stop doing work (which is impossible), they I will stop using ATP into ADP, so the amount of ADP available to make more ATP will be reduced, therefore the rate of proton entering the matrix will slow down and the proton gradient will be higher and higher and the proton pumping will slow down which makes ET chain slow down, The NADH and FADH2 will need to wait longer to drop the H and e-, and therefore they won't be converted back into NAD+ and FAD as quickly, so they will burn the fuels more slowly.
What is the general structure of fatty acids? What are some of its features?
The general structure: CH3CH2CH2CH2CH2CH2CH2CH2CH2——CH2CH2COOH
Features:
1. Nearly all the carbon atoms are fully reduced
2. Stored as triglyceride
3. Totally hydrophobic
4. Very energy dense (37 kJ/g)
5. Huge stores (many kg)
6. Can’t be used by brain
Fatty acids are trapped in cytoplasm as what?
Fatty acids are transported into mitochondria via what carrier?
The H/e- of fatty acids are ripped out by what?
Fatty acid part loses what chunk?
Fatty acids are trapped in cytoplasm as Fatty Acyl-CoA.
Fatty acids are transported into mitochondria via carnitine as the carrier.
The H/e- of fatty acids are ripped out by FAD and NAD+.
Fatty acid part loses an acetate chunk.
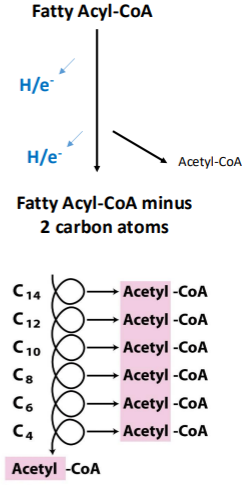
Carnitine is sold as a fat metaboliser… would it work?
Carnitine is a naturally occurring compound that helps transport long-chain fatty acids into the mitochondria, the energy-producing parts of cells, where they are burned for fuel. The idea behind carnitine supplements is that by enhancing fat transport into the mitochondria, it could boost fat burning and aid in weight loss. However, research has shown that the effects are minimal for most people, especially when taken without exercise. Some studies suggest that carnitine may help increase fat oxidation during endurance activities, but it’s not a significant fat loss tool on its own. For real results, a combination of proper diet and regular physical activity is essential.
What is the general structure of glucose? What are some features of glucose and glycogen?
Features:
Reasonably reduced
Stored as glycogen
Hydrophilic, lots of water associated
Inefficient; 16 kJ/g (but only 6 kJ/g wet)
Low stores (300 g)
Can be used by all tissues
Brain has an obligatory requirement!
Glucose are found in the bloodstream and inside cells.
Most of the Glycogen are stored in our livers and muscles.
How is glucose trapped inside the cell
Hexokinase adds a phosphate to glucose so it is trapped inside the cell.
What are some features of glycolysis
All tissues
Wholly cytosolic
No requirement for oxygen
Very, very fast
Very, very inefficient
ATP generation almost irrelevant (with some BIG exceptions) compared to oxidative phosphorylation
Pyruvate must be transported into mitochondria for full oxidation
What would happen if we stored all our fuels as glycogen?
If all our fuel were stored as glycogen instead of a mix of glycogen and fat, our bodies would be much heavier due to glycogen’s high water retention (about 3g of water per gram of glycogen), making movement inefficient. Glycogen is also rapidly depleted, lasting less than a day, whereas fat provides long-term energy storage. Since fat is more energy-dense (9 kcal/g vs. 4 kcal/g for glycogen), relying only on glycogen would require constant eating to maintain energy levels. Additionally, glycogen’s quick conversion to glucose could cause frequent blood sugar spikes, leading to metabolic imbalances.
What are some features of using protein as fuels
Many pathways for amino acid catabolism
Channel into pyruvate, acetyl-CoA or Krebs
Energy release about 17 kJ/g
Need to dispose of amine groups
‘store’ is about 5-10 kg
Protein is last ditch fuel store
We don’t ‘store’ protein – it all has a function
Making protein has cost us a LOT of energy
Metabolism and fire both have the general equation fuel + oxygen → CO2 + water, what is the difference between these two.
One difference is fire is supply driven: more you put in, bigger the fire and metabolism is demand driven.
L02
What fuel do we mainly burn when we go for a 20 min run?
a) Fats
b) Glucose
c) Proteins
d) Acetyl CoA
a) Fats
What are two types of muscle cells we talked about in class
Type I - “Red”, “Slow”
– Contracts relatively slowly
– Many mitochondria
– Good blood supply
Type IIb -“White”, Fast”
– Contracts relatively rapidly
– Few mitochondria
– Poor blood supply
– Packed full of contractile filaments
What will happen when we initiate exercise?
ADP concentration increases as ATP turns into ADP.
More ADP available for ATP synthase and more protons can re-enter the mitochondrial matrix.
The proton gradient gets smaller so more H and e- can enter the electron transport chain.
NADH and FADH2 can drop off H and e- and can be regenerated into NAD+ and FAD more frequently.
NAD+ and FAD can go and burn more fuels more frequently.
ATP trunover is what in relation to stores (even at rest)?
ATP turnover is very high in relation to stores even at rest
• ’store’ 1 g/kg body weight – levels held in very narrow range
• ‘turnover’ 1 kg/kg body weight
If i weigh 70 kg, I have 70 grams of ATP, but every day I am going to turn over around 70 kg of ATP (turn ATP into ADP or AMP and back to ATP again).
What will happen when we start gentel exercise
The most readily available fuel is glucose, so the glucose transporter takes glucose into the cell and start the oxidation will start. This will lower blood glucose and we need to keep that at 5 mM for our brains.
Tiny decrease in blood glucose gives big hormonal responses and the insulin will decrease and glucagon will increase. Glucagon will do two things: first it will stimulate glycogen breakdown into glucose in liver and we can release that into the bloodstream; second it will stimulate fat breakdown in white adipose tissue and the fatty acids will be released into bloodstream.
Glucose stores (glycogen) are limitted and we cannot convert fatty acids into glycose. So fatty acids will substitute for glucose as a fuel and prevent glucose from bing wastefully oxidised. If excess fatty acids provide more acetyl-CoA than the Krebs cycle needs, the buildup inhibits pyruvate dehydrogenase, preventing pyruvate from converting to acetyl-CoA. Instead, incoming glucose is converted to lactate, which can be sent to the liver for glucose regeneration.
Once we get from 3-carbon Pyruvate to 2-carbon acetyl-CoA, the glucose is now lost forever. But if we can stop it here at pyruvate and convert it to lactate, it can be returned to the liver where it can be turned back into glucose again.
In summary:
– Initially, glucose is used
– After several minutes fatty acids take over (released from White Adipose tissue)
– Glucose still gets into the muscles, but it is only taken as far as lactate
– Lactate goes to the liver for re-synthesis of glucose (Gluconeogenesis)
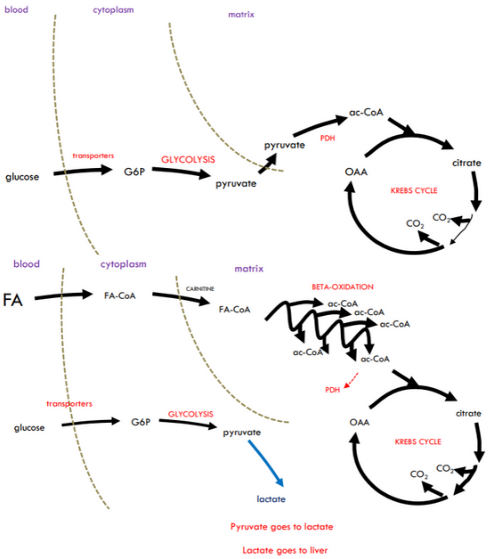
What will happen when we jump from gentle exercise to moderate exercise?
As the pace increases: the rate of fatty acid utilisation increases, but the enzymes that catalyse fatty acid oxidation soon reach their maximum capacity. So fatty acid oxidation alone cannot maintain ATP production: Inhibition on glucose oxidation is removed. Glucose oxidation occurs: Less glucose recycling and Liver glycogen stores depleted faster.
A mixture of fatty acid oxidation and glucose oxidation (The glucose comes from the liver). Any further increase in pace met by increase in glucose oxidation (Fatty acid oxidation going at full speed already)
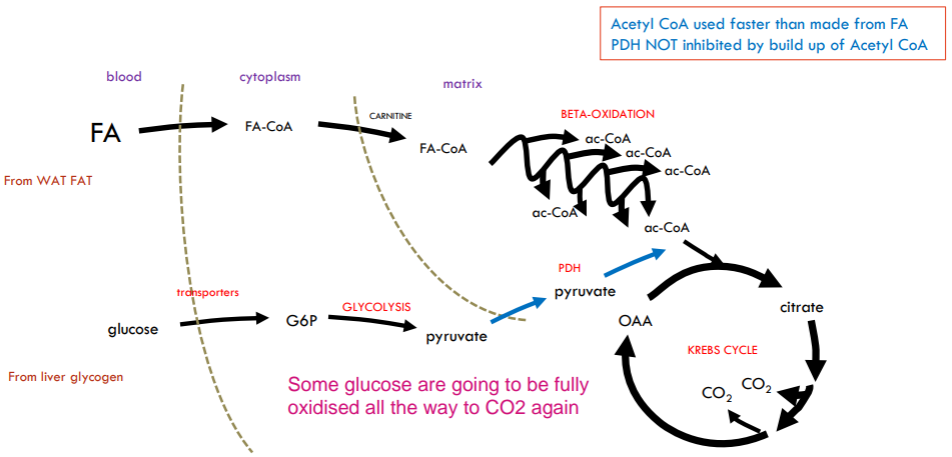
What will happen when we jump from moderate exercise to strenuous exercise?
Now limits on speed of oxidation of blood glucose: rate of supply and transport from blood cannot keep up while fatty acids still going as fast as they can.
So the endogenously stored muscle glycogen start to be broken down.
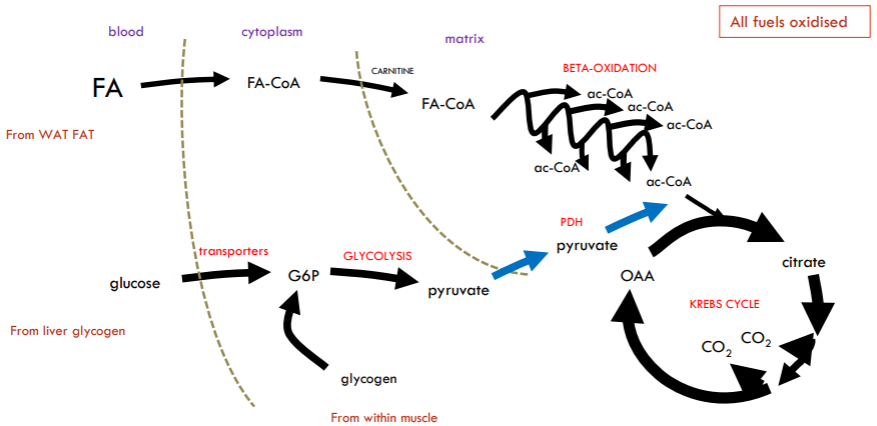
What will happen when we jump from strenuous exercise to very strenuous exercise?
Now rate of ATP production can’t be met by oxidative phosphorylation alone (Mitochondrial processes too slow). Need to top up with extra boost from glycolysis (glycolysis is VERY fast and Very inefficient and now blood lactate levels will rise). Glucose must come from muscle glycogen asa transport already at max.
During very strenuous exercise, glycogen is converted to lactate because the muscles require ATP faster than oxygen can be delivered to support aerobic respiration. As a result, cells rely on anaerobic glycolysis, which rapidly breaks down glycogen into glucose and then to pyruvate. However, without sufficient oxygen, the mitochondrial electron transport chain cannot regenerate NAD+ from NADH efficiently. To sustain glycolysis, pyruvate is converted into lactate by lactate dehydrogenase, which regenerates NAD+. This allows ATP production to continue in the absence of oxygen, but it also leads to the accumulation of lactate in the muscles.
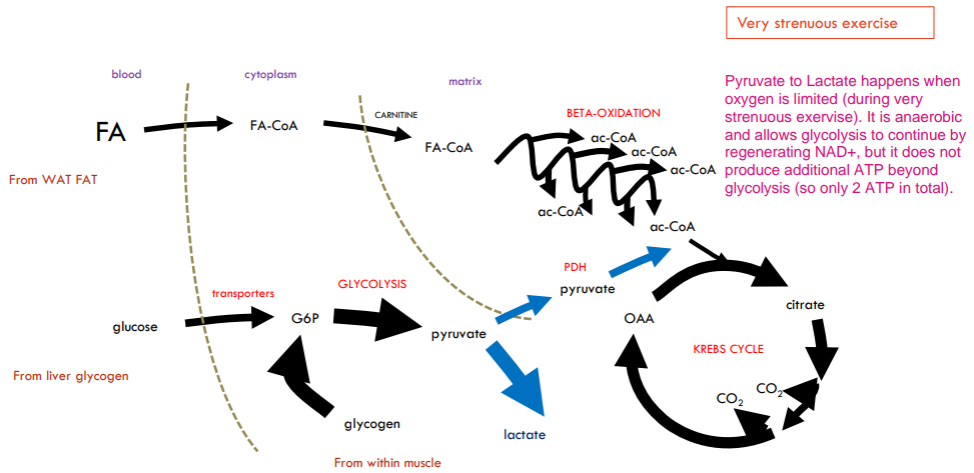
What will happen during sprinting
During sprinting, the body primarily relies on Type IIb muscle fibers, which are specialized for short, explosive bursts of power. These fibers have a poor blood supply, contain few mitochondria, and are densely packed with contractile filaments, enabling rapid and forceful contractions. Sprinting demands very rapid ATP consumption, but these fibers face a fuel selection problem: fatty acids cannot be used due to the low oxygen availability and mitochondrial content, and blood glucose is ineffective in the short term because of delayed glucose transporter activation and limited immediate supply. As a result, Type IIb fibers rely almost entirely on glycolysis of stored glycogen, which rapidly converts glucose to lactate. While glycolysis is fast, it is also highly inefficient, producing only 2 ATP per glucose molecule, compared to over 30 via full oxidation. Furthermore, this rapid glycolysis must continually regenerate (H/e carrier) NAD⁺ to keep running, leading to the accumulation of large amounts of lactate in a short time. Due to the poor blood supply, this lactate cannot be cleared efficiently, contributing to muscle fatigue and the burning sensation often felt during intense sprints.
What is Glycogenolysis
Glycogenolysis is the process by which glycogen in the liver and muscles, is broken down into glucose to be used for energy.
What can Creatine Phosphate (CP) do?
CP is an instant store of ATP, but less than five seconds supply (15 mM).
creatine phosphate + ADP → ATP + creatine.
Creatine supplements boost CP levels.
Anaerobic Glycolysis: Your muscles use stored glycogen to produce ATP rapidly through glycolysis, converting glucose into pyruvate and then lactate (if oxygen is limited). This provides quick bursts of energy.
Stored ATP & Phosphocreatine (PCr): Your muscles already have a small amount of stored ATP and phosphocreatine (PCr), which can power movement for 5-10 seconds without needing oxygen
Can you run a sprint without breathing?
Yes, you can technically run a short sprint without breathing, but only for a limited time. During an all-out sprint (like a 100m dash), your muscles rely heavily on anaerobic metabolism, meaning they generate energy without needing oxygen.
A lecture question is at the last slide of this lecture. I did not add it because I don’t know what the quiz is.
L03
Do we make any ATPs during beta-oxidation? What are some of the things we do during this process?
We don’t make any ATPs during beta-oxidation. We make lots of NADH and FADH2 from NAD+ and FAD. We also build ac-CoA which will enter kreb cycle (which will give us even more NADH and FADH2).
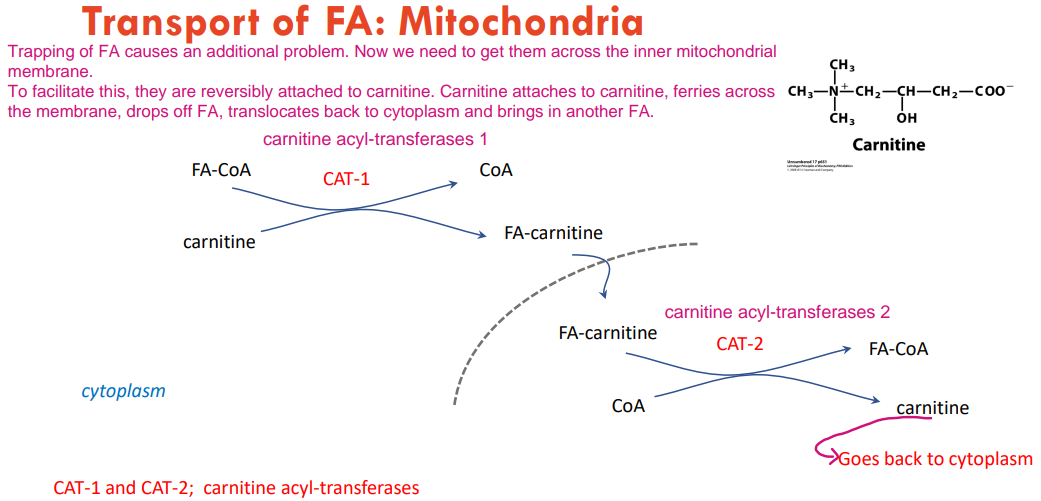
How do we help FA-CoA to enter the matrix from the cytoplasm?
We take the CoA off and use carnitine that will help them into the mitochondria matrix. We will swap the carnitine back to CoA and turn it back to FA-CoA after they enter the matrix.
Why do biochemists call beta-oxidation beta-oxidation?
Because the β-carbon atom is where NAD+ and FAD do their work and strip the H and e- from the fatty acid chain.
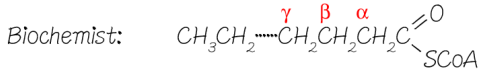
Fatty acids can loosely associated with what? Why is that?
Due to similar reason, they bind to what in cytoplasm until they do what?
Fatty acids can loosely associated with albumin. Fatty acids are quite hydrophobic (not very soluble in plasma). They act like soap because they have hydrophobic and hydrophilic parts. They can bind to to albumin which helps to make them more soluble in plasma.
Fatty acids bind to Fatty acid binding protein (FABP) when they are in cytoplasm until they are ultimately tagged (trapped) with CoA.
Do fatty acids passively diffuse acrom cell membrane or do they require active transport?
Mostly is passive diffusion.
How are fatty acids trapped with CoA inside cytoplasm?
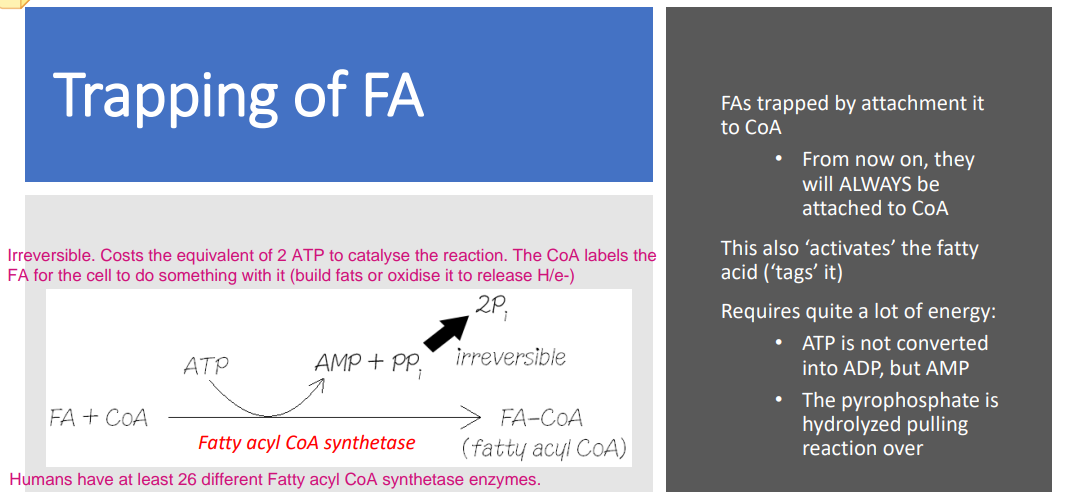
The acyl group (including fatty acyl) binds to which group of the CoA.
The acyl group binds to sufhydryl group (-SH) which is part of the β-Mercapto-ethylamine unit. The sufhydryl group (-SH) is the reactive site.
How are FA-CoA transfered intot the mitochondrial matrix from cytoplasm?
After each round of beta-oxidation, the fatty acid carbon chain is how many carbons shorter?
What do we get after each round of beta-oxidation?
After each round of beta-oxidation, the fatty acid carbon chain is 2 carbons shorter.
We get 1 acetyl CoA, 1 NADH and 1 FADH2.
For a C16 fatty acid:
How many times do you need to break a C16 backbone to produce all C2 fragments?
How many acetyl CoA molecules will you get?
How many NADH will you get?
How many FADH2will you get?
7
8
7
7
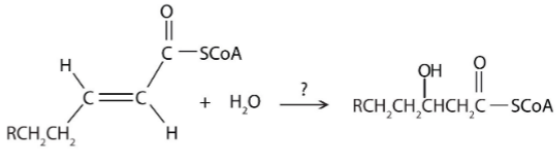
What type of reaction is shown here?
A. Oxidation-reduction
B. Hydration
C. Cleavage
B. Hydration
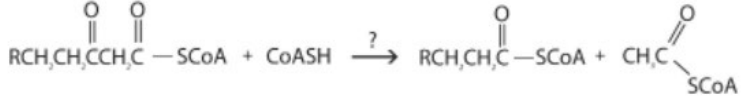
What type of reaction is shown here?
A. Oxidation-reduction
B. Hydration
C. Cleavage
C. Cleavage
A cleavage reaction refers to a type of chemical reaction in which a larger molecule breaks down into smaller fragments or components.
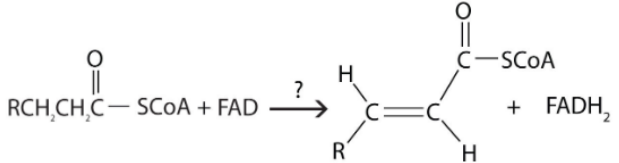
What type of reaction is shown here?
A. Oxidation-reduction
B. Hydration
C. Cleavage
A. Oxidation-reduction
If you see FAD or NAD in a reaction, it's probably an oxidation reduction reaction.
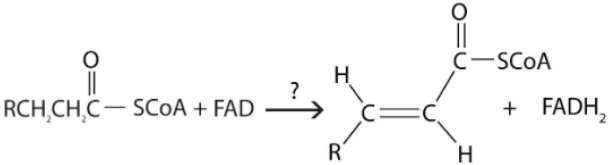
What has been reduced?
A. FA-CoA
B. FAD
B. FAD
How do we transport glucose from the blood to the cytoplasm?
Unlike fatty acids, glucose cannot just diffuse into the cell, we need Glucose transporters (GLUTs).
GLUT-1 present in all cells all the time
GLUT-4 muscle and adipose tissue (the insulin sensitive tissues)
GLUT-2 liver and pancreas (blood glucose regulating tissues)
After going into teh cytoplasm, the glucose can still go out. So we transformed it into what?
We transformed it into glucose 6-phosphate (G6P) and this cannot go out.
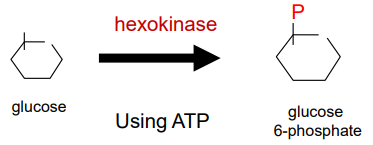
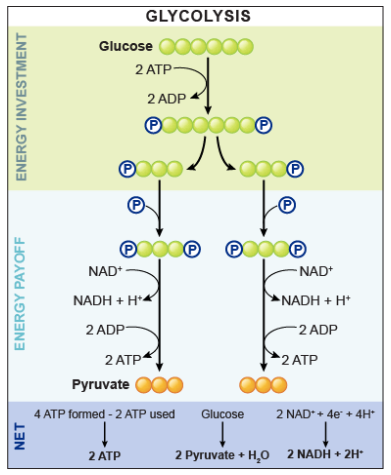
During the early glycolysis, what do we need to do.
During the early glycolysis, we need to put energy in, which will give a bi-phosphorylated symmetrical sugar. We will further break it in half and get two 3-carbon molecules. We use 2 ATP in this stage.
How many ATPs do we get during the late glycolysis?
4 ATPs
What is the total yield of glycolysis?
2 ATPs, 2 pyruvate and 2 NADH.
What are two main fates of pyruvate after glycolysis:
1. Aerobic conditions
Pyruvate is transported into the mitochondria.
It is converted into acetyl-CoA during the decarboxylation reaction.
Acetyl-CoA then enters the Krebs cycle (TCA cycle).
This leads to production of more NADH, FADH₂, and ATP through the Krebs cycle and oxidative phosphorylation.
2. Anaerobic conditions
Pyruvate is converted to lactate by the enzyme lactate dehydrogenase.
This regenerates NAD⁺ so that glycolysis can continue producing ATP even without oxygen.
For cells without mitochondria like blood cells, they have to rely on what?
For cells without mitochondria like red blood cells, they have to rely on glycolysis after which the pyryvate is turned into lactate so that they can perform glycolysis again.
A kinase is an enzyme that:
A. removes phosphate groups from substrates
B. uses ATP to add a phosphate group to the substrate
C. uses NADH to change the oxidation state of the substrate
D. removes water from a double bond
B. uses ATP to add a phosphate group to the substrate
Which of the following is INCORRECT? The pyruvate produced in glycolysis…
A. Is further oxidised in the Krebs Cycle
B. Is decarboxylated by pyruvate dehydrogenase to give acetyl CoA
C. Is oxidised to lactate to regenerate cytosolic NAD+
D. Is a 3-carbon molecule
A. Is further oxidised in the Krebs Cycle
A is incorrect because pyruvate itself does not directly enter the Krebs Cycle. First, it is converted into Acetyl-CoA by the pyruvate dehydrogenase complex before it enters the Krebs Cycle.
What is the substrate for the Krebs Cycle (citric acid cycle) and where does it come from.
The substrate is acetyl CoA from fatty acid oxidation and/or glucose oxidation.
Where does the kerebs cycle (citric acid cycle) take place?
The krebs cycle takes place in mitochondria.
What is the overall strategy of krebs cycle (citric acid cycle)
Completely oxidise acetate carbons to CO2
Produce lots of NADH, FADH2, even some ATPs (…not directly)
Performing the reactions on a carrier molecule
What is the process of krebs (citric acid) cycle?
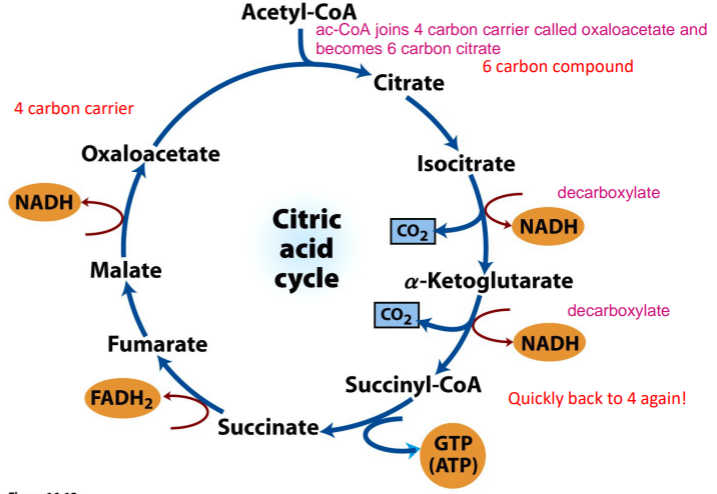
During each krebs cycle (citric acid cycle), what comes in and what comes out?
Acetyl CoA (2 carbon) comes in and it is turned into 2 CO2. 3 NADH,1 FADH2, and 1 GTP are also generated. The Acetyl CoA is not converted into oxaloacetate or sucinates. Oxaloacetate is not ‘net’ consumed in the cycle, it acts as carrier.
Each NADH give 2.5 ATP and each FADH2 gives 1.5 ATP in oxidative phosphorylation (in ETC). So with the GTP, that is about 10 ATP per acetyl-CoAn but the ATP is not generated directly!!! The purpose of the kreb cycle is not to make ATP.
Generally, the more of what, the faster the fuel oxidation?
Generally, the more NAD+, FAD and ADP, the faster the fuel oxidation?
Generally, if there is NAD+ and FAD available, we're going to burn some fuel. Whenever we want to make ATP we're also going to need some ADP to make that ATP. And if we can't make ATP we can't burn the fuels.
L04
What are the consequences of No Proton gradient
No driving force for ATP synthesis
Instant regeneration of NAD from NADH
No ATP synthesis which means cell death
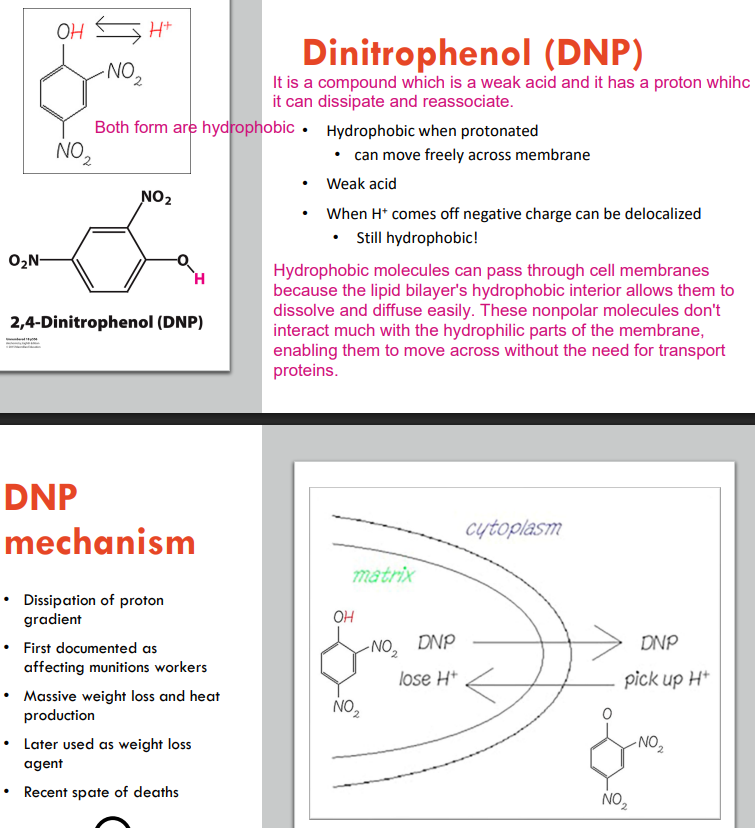
How Dinitrophenol (DNP) can disrupting the metabolism?
It allows you to short circuit that mitochondria and allow the protons to flow back in without actually forming any ATP. Essentially, you can burn lots of fuels without making any ATP or doing any work. You can have shortness of breath or overheat while you are sitting by taking this kind of drugs.
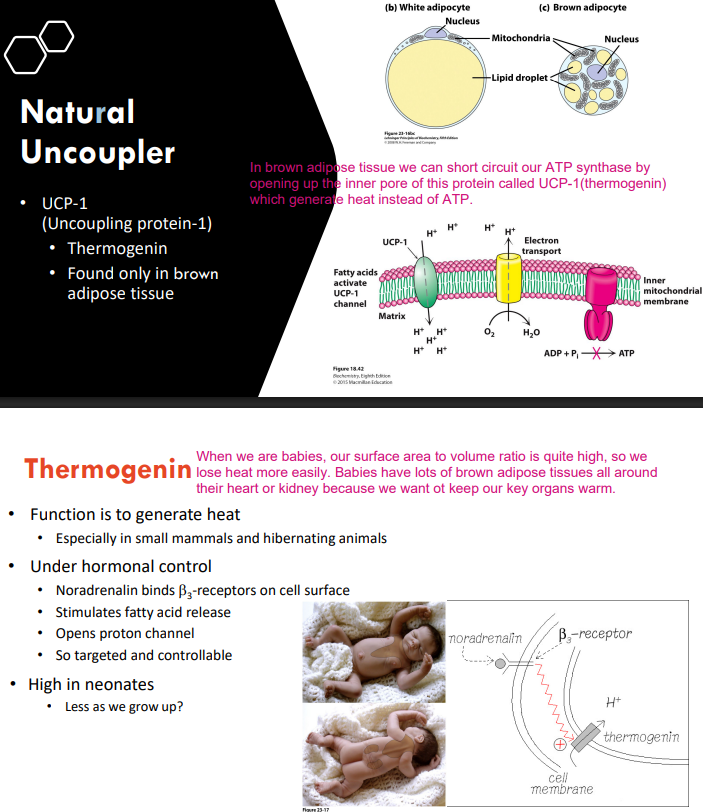
How does natural uncoupler work?
What are the five (the slide says four) complex embeded in the inner mitochondrial membrane for the electron transport chain?
Which one does not pump protons?
The protons pumped out are from where?
Complex I, II, Q, III, and IV.
Complex II does not pump protons, while the other four do.
The protons pumped out are from the matrix and not from the NADH or FADH2.
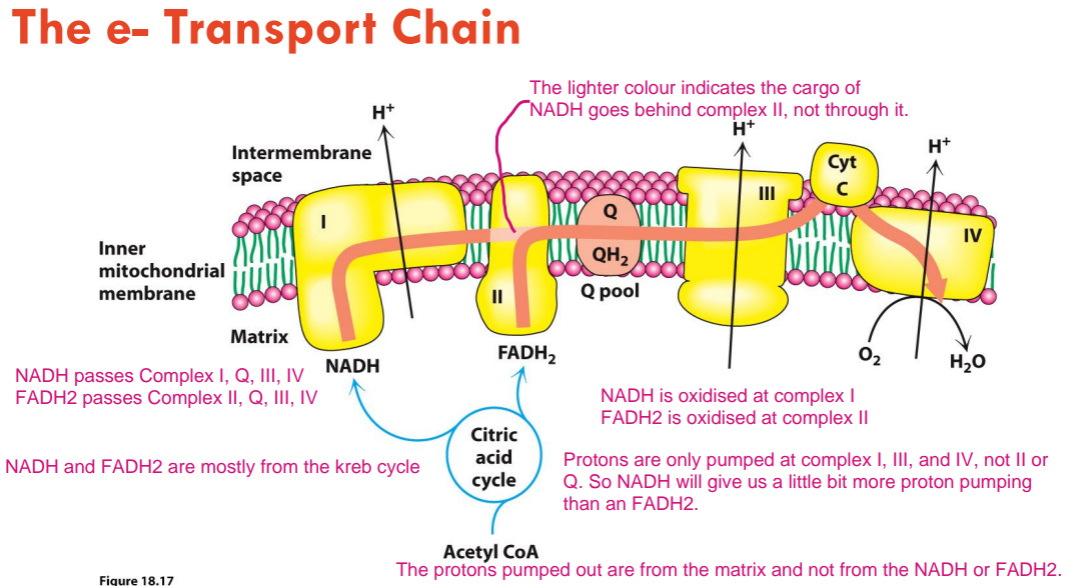
NADH passes which complexes? What about FADH2? Where are they oxidised (where do they donate their H/e-)? Roughly how many protons are pumped out for each NADH?
NADH passes complex I, Q, III, IV.
FADH2 passes complex II, Q, III, IV.
NADH is oxidised (donates H/e-) at complex I and FADH2 is oxidised (donates H/e-) at complex II.
Protons are only pumped at complex I, III, and IV, not II or Q. So NADH will give us a little bit more proton pumping than an FADH2.
Around 10 protons are pumped out for each NADH.
Each complex in ETC consists of what? Proteins are arranged that
Each complex consists of many proteins
• Structural - maintain shape of complex
• Prosthetic group – bits that transport H/e-
Proteins are arranged so that:
• H+ expelling reactions are on the outside
• H+ consuming reactions are on the matrix side
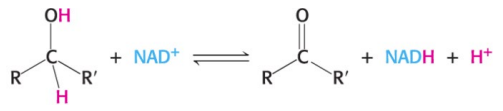
How many hydrogen and electrons does NAD+ accept?
NAD+ accepts a hydrogen ion and two electrons (equivalent to a hydride ion H-) (he other H+ is in left in the solution, so it is not called NADH2)
How many hydrogen and electrons does FAD accept?
FAD is totally stuck in what structure?
FAD accepts 2 H+ and 2 electrons.
Unlike NAD+ or NADH which can float around, FAD or FADH2 are actually part of (stuck in) the complex II of the electron transport chain. So anyhing includes FAD or FADH2 like beta oxidation or one of the steps in the Kreb cycle that gives us some FADH2 is happening at complex II.
What are some features of UQ (Ubiquinone)
It is also named coenzyme Q, Q10, etc.
Electrons move around in Complex I form one prosthetic group to another (sometimes with protons, sometimes not!) until they reach the Q pool.
UQ is very hydrophobic. It lives in inner mitochondrial membrane. It is often added in shampoos and things like that.
UQ also picks up hydrogens from Complex II.
Reduced UQ is UQH2.
UQH2 transfers hydrogens to Complex III.
UQ is also sold as an erogenic compound which might increase athletic abilities by bolstering your ETC.
What are some features of Cytochrome C (Cyt C) and Iron.
Cyt C picks up electrons from Complex III and gives electrons to Complex IV.
Cyt C has a prosthetic group which contains an iron atom. The iron atom changes from ferrous to ferric as it loses and, vice versa, as it accepts the electrons. Iron does NOT carry hydrogens. Every single part of the electron transport chain transport electrons, and they don’t carry hydrogens.
Cytochrome C and Iron only like to deal with electrons.
What is the mechanism of proton pumping?
You are a CARRIER of HYDROGENS. This means you are carrying A PROTON plus AN ELECTRON (because that’s what a H is!!!)
Imagine you BUMP INTO a carrier of ELECTRONS. That is, something that CARRIES ELECTRONS but DOESN’T WANT TO DEAL WITH PROTONS.
SO, you’ve gotten rid of your cargo.
THEY have their ELECTRON.
What gets RELEASED is a PROTON!
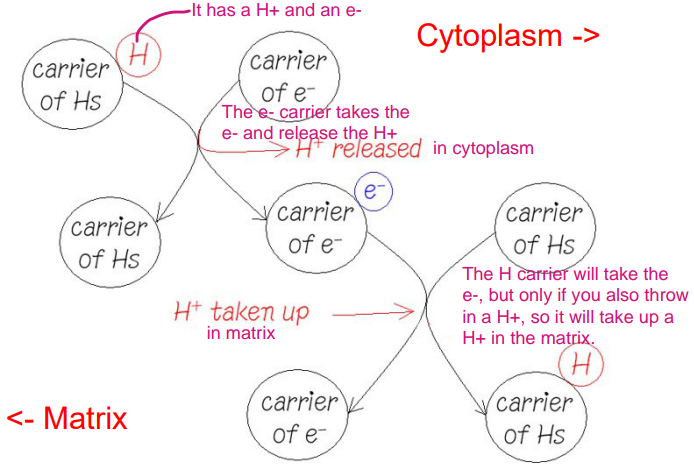
How many protons are pumped out for each NADH and FADH2
10 Protons are pumped out for each NADH (4 at complex I, 4 at complex III and 2 at complex IV) and 6 protons for each FADH2 (4 at complex III, and 2 at complex IV).
How many lipid bilayer membranes does the mitochondrion have?
When mitochondria is pumping protons out of the matrix, where does it pumping protons out to?
The mitochondrion has two lipid bilayer membranes.
The mitochondion is pumping protons to the intermembrane space between the outer mitochondrial membrane and the inner mitochondrial membrane.
The proton pump in ETC builds up a electrochemical gradient. What are two components of the gradient?
It is both a chemical gradient and an electrical gradient. More H+ (chemical) and positive charge (electrical) on the p side.
How does the NADH generated in glycolysis get in from the cytoplasm to the matrix. (NADH needs to drop proton and e- in the matrix)
Glycerol 3-Phosphate shuttle. (This by-passes complex I, so we get 6 protons pumped per NADH for these NADHs instead of 10.)
Malate Aspartate shuttle. (NADH transfered without loss of proton pumping potential)
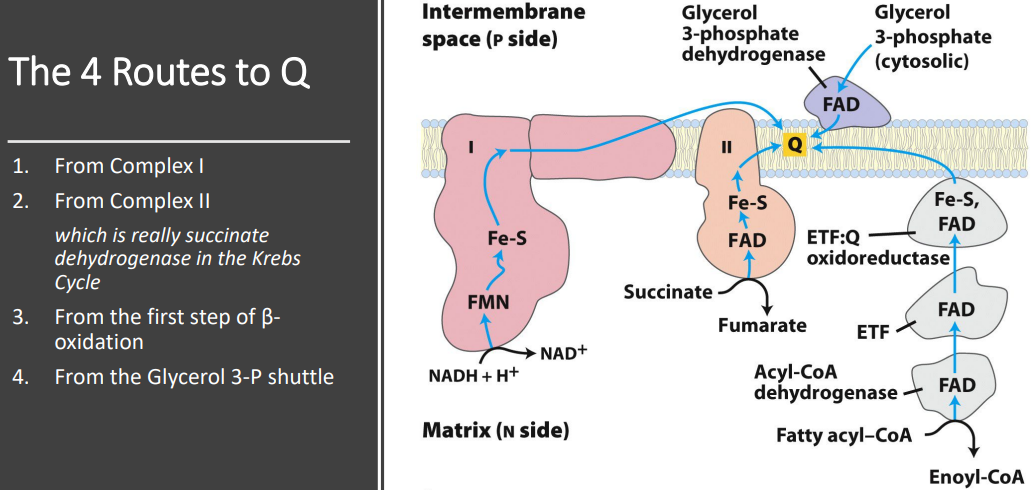
What are the four pathsway for electrons to go to UQ
Electrons enter the ubiquinone (Q) pool through four main pathways: from 1. NADH via Complex I, 2. FADH₂ via Complex II, 3. fatty acid β-oxidation via ETF:Q oxidoreductase, and 4. cytosolic NADH via the glycerol-3-phosphate shuttle.
Electrons from NADH pass through a flavoprotein to a series of iron-sulfur proteins (in Complex I) and then to Q.
Electrons from succinate pass through a flavoprotein and several Fe-S centers (in Complex II) on the way to Q.
Acyl-CoA dehydrogenase (the first enzyme of β oxidation) transfers electrons to electron-transferring flavoprotein (ETF), from which they pass to Q via ETF: ubiquinone oxidoreductase.
Glycerol 3-phosphate donates electrons to a flavoprotein (glycerol 3-phosphate dehydrogenase) on the outer face of the inner mitochondrial membrane, from which they pass to Q.
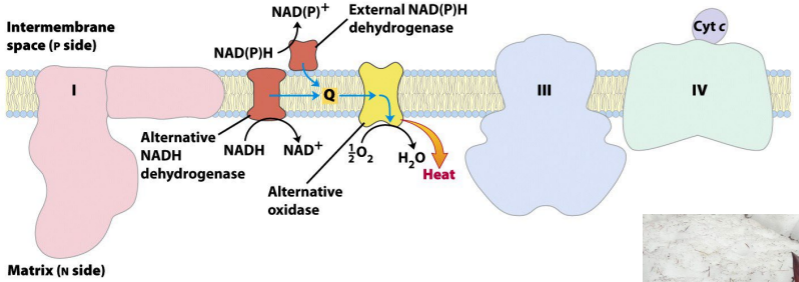
How does skunk cabbage uncouple?
Electron carriers of the inner membrane of plant mitochondria. Electrons can flow through Complexes I, III, and IV, as in animal mitochondria, or through plant-specific alternative carriers by the paths shown with blue arrows.
Anything that moves electrons without forming ATP is going to GENERATE HEAT.
This system sends the HYDROGENS almost DIRECTLY to OXYGEN. That’s just like a H-BOMB or firing a ROCKET to the MOON.
MOST of the H/e are taking the NORMAL PATHWAY. Just a few are ALLOWED to take this ALTERNATIVE PATH via Q.
The features of free radicals and the formation of free radicals.
Electrons in the UQ pool can react with molecular oxygen which can produce free radicals.
Free radicals are very dangerous as it can cause mutations to DNA.
Free radicals are less likely to form if Complex III is vacane. Free radicals forming is more likely to happen if there is a trafic jam or bank up in the ETC, and is less likely to happen if everything is flowing smoothly.
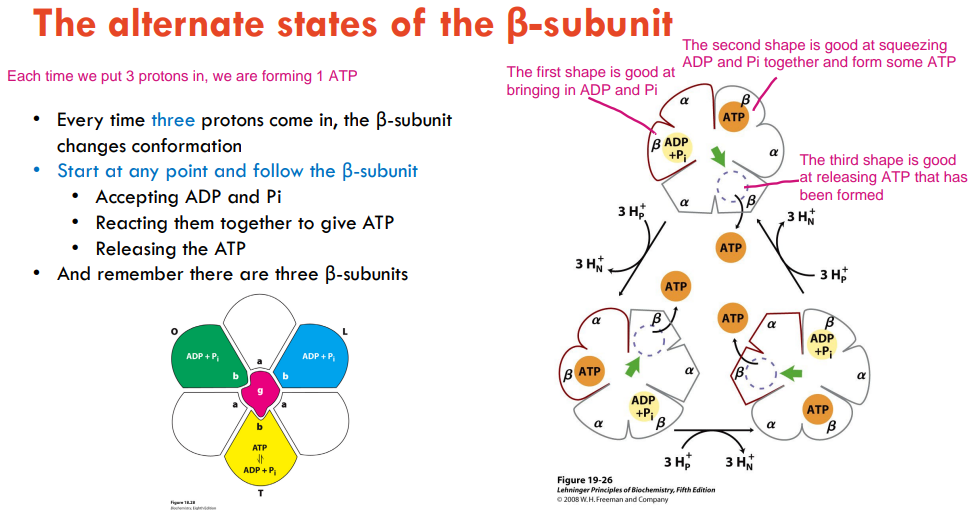
The movement of how many protons can generate 1 ATP?
Why don’t we get 3 ATP for each NADH?
The movement of 3 protons generates 1 ATP.
So we would expect to get around 3 ATP for each NADH because each NADH pumps out 10 protons, but it is actually more like 2.5 because we USE the GRADIENT for some OTHER STUFF.
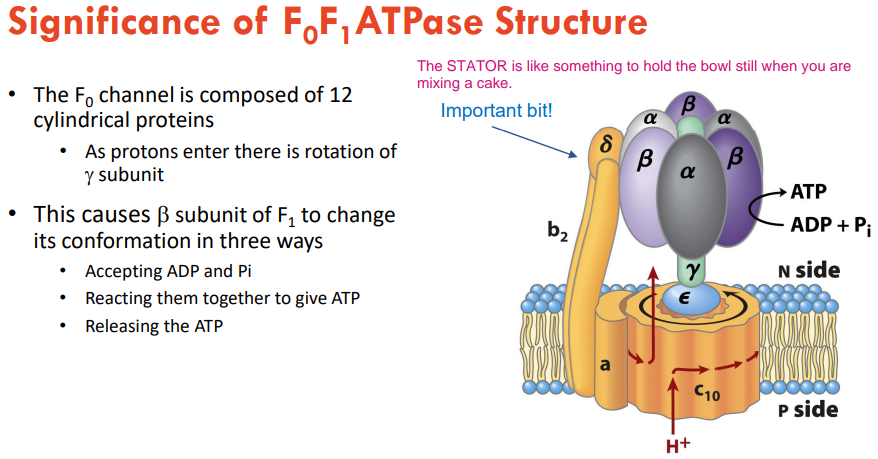
What are the significances fo F0 F1 ATPses structure
How does β-subunit work?
How proton gradient is also used in transport?
The proton gradient across the inner mitochondrial membrane supports transport by driving phosphate (H₂PO₄⁻) import along with H⁺ and by favoring ATP export and ADP import through the adenine nucleotide translocase, which relies on the charge difference. This ensures a steady supply of substrates for ATP synthesis and efficient export of ATP to the cytosol.
L05
When does the starvation start?
Starvation begins at the start of the post-absorptive period when all food is digested, no substrates are coming in from gut and our bodies rely on blood and stored fuels.
What is hyperglycemic, hypoglycemic and euglycemic.
Hyperglycemic: very high blood glucose concentration
Hypoglycemic: very low blood glucose concentration
Euglycemic: the right amount of blood glucose concentration