Chemistry - chapter R1.1 to 1.4 (excluding 1.3.5 and 1.4.4)
1/30
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
31 Terms
lattice enthalpy
exothermic when applied to the formation of the lattice: aMb+(g) + bXa-(g) —> MaXb (s)
endothermic when applied to the breakdown of the lattice: MaXb (s) —> aMb+(g) + bXa-(g)
ionization enthalpy
M(g) —> M+(g) + e-, endothermic
specific energy
energy per unit mass (energy released from the fuel/mass of the fuel consumed
heat
the energy that flows from something at a higher temperature to something at a lower temperature because of the temperature difference between them
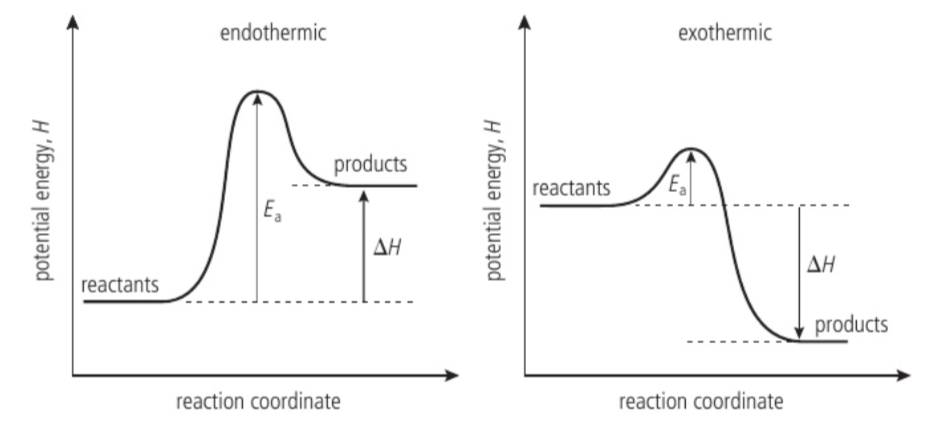
exothermic reaction
a chemical reaction that results in the release of heat to the surroundings - the reaction vessel gets hotter; ΔH for an exothermic reaction is negative
endothermic reaction
a chemical reaction in which heat is taken in from the surroundings - the reaction vessel gets colder; ΔH for an endothermic reaction is positive
enthalpy change ΔH
the heat energy exchanged with the surroundings at constant pressure
system/surroundings
system refers to the chemicals themselves, whereas the surroundings refers to the solvent, the air and the apparatus all that surrounds the chemicals
internal energy (sometimes called chemical energy)
the name given to the total amount of energy (kinetic and potential) in a sample of a substance
potential energy profile diagram
a diagram showing the change in the potential energy (y-axis) of a system as a reaction proceeds (x-axis is the reaction coordinate)
calorimetry
experimental determination of the heat given out/taken in during chemical reactions/physical processes
specific heat capacity
the energy required to raise the temperature of 1 g of substance by 1 K (1 °C). It can also be defined as the energy to raise the temperature of 1 kg of substance by 1 K. Specific heat capacity has units of J g-1 K-1 or 1 g-1 °C-1. Units that are also encountered are kJ kg-1 K-1 or kg-1 K-1
standard enthalpy change of neutralization (ΔHn)
the enthalpy change when one mole of H2O molecules is formed when an acid (H+) reacts with an alkali (OH-) under standard conditions, i.e H+(aq) + OH(aq) → H2O(l) the enthalpy change of neutralization is always exothermic
bond enthalpy
the enthalpy change when one mole of covalent bonds, in a gaseous molecule, is broken under standard conditions. Bond breaking requires energy (endothermic), ΔH is positive; bond making releases energy (exothermic), ΔH is negative
Hess’s law
the enthalpy change accompanying a chemical reaction is independent of the pathway between the initial and final states
average bond enthalpy
the average amount of energy required to break one mole of covalent bonds, in a gaseous molecule under standard conditions; 'average' refers to the fact that the bond enthalpy is different in different molecules and, therefore, the value quoted is the average amount of energy to break a particular bond in a range of molecules
standard enthalpy change of combustion ΔH⦵c
the enthalpy change (heat given out) when one mole of a substance is completely burnt in oxygen under standard conditions
standard enthalpy change of formation ΔH⦵f
the enthalpy change when one mole of a substance is formed from its elements in their standard states under standard conditions. ΔH⦵f for any element in its standard state is zero
Born-Haber cycle
an enthalpy level diagram breaking down the formation of an ionic compound into a series of simpler steps
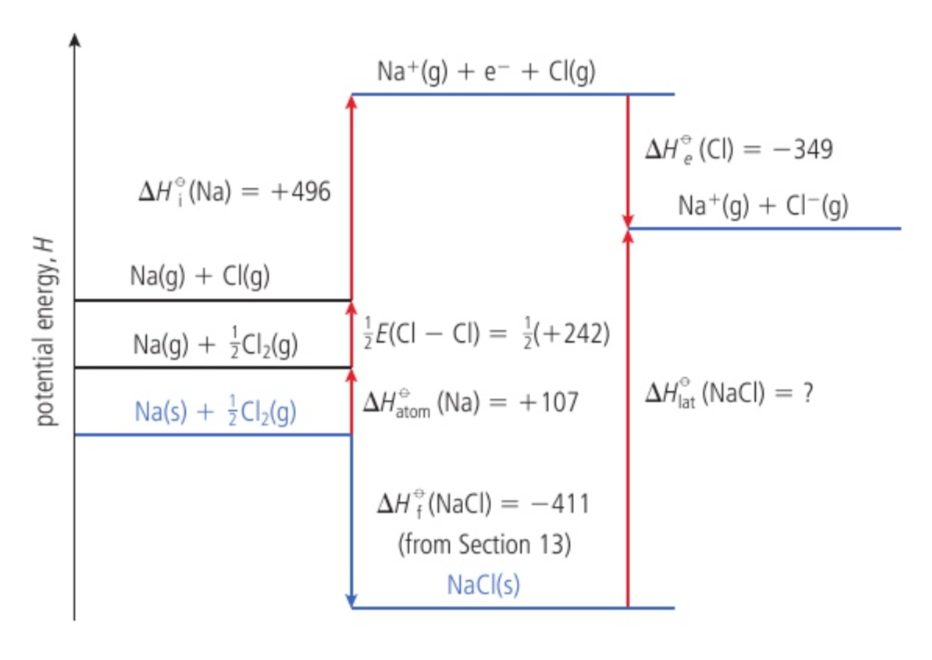
standard enthalpy change of atomization ΔH⦵at
the enthalpy change when one mole of gaseous atoms is formed from an element in its standard state under standard conditions; endothermic
first electron affinity
enthalpy change when one electron is added to each atom in one mole of gaseous atoms under standard conditions: X(g) + e- —> X-(g) The first electron affinity is exothermic for virtually all elements
second electron affinity
the enthalpy change for the following process: X-(g) + e- —> X2-(g)
combustion
burning, an exothermic reaction that occurs when a substance reacts with oxygen. Usually these reactions produce a flame and continue once the initial heat source is removed
complete combustion
the burning of a substance in a plentiful supply of oxygen
incomplete combustion
the burning of a substance in a limited supply of oxygen
fuel
something that is burnt to produce energy
renewable energy sources
sources of energy that are naturally replenished they will not run out, e.g. solar energy or wind power
non-renewable energy sources
Sources of energy that are finite - they will eventually run out, e.g. coal
biofuels
Renewable fuels derived from organic matter like plants and algae. Can be used as an alternative to fossil fuels for energy production, reducing greenhouse gas emissions.
entropy (S)
a measure of the disorder of a system (how the matter is dispersed/distributed) or how the available energy is distributed among the particles. Standard entropy (S) is the entropy of a substance at 100 kPa and 298 K; units are JK-1 mol-1. ΔS is the entropy change under standard conditions a positive value indicates an increase in entropy, i.e. the system becomes more disordered/the energy becomes more spread out (less concentrated)
Gibbs energy change ΔG or free energy change
ΔG is related to the entropy change of the universe and can be defined using the equation ΔG = ΔΗ - TΔS. For a reaction to be spontaneous, ΔG for the reaction must be negative. ΔG is the standard energy change