1.5 b/ OZ 1- Gas Concentrations and Atmospheric Composition
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Define atmosphere
A relatively thin layer of gas that surrounds the earth's surface which extends 100 km above the Earth's surface which has a big influence on the Earth
What keeps the atmosphere in place?
Held in place by gravity acting downwards towards the Earth's surface
What does the atmosphere look like?
Seen as a thin blue haze around the Earth
Draw a diagram of the layers of the atmosphere
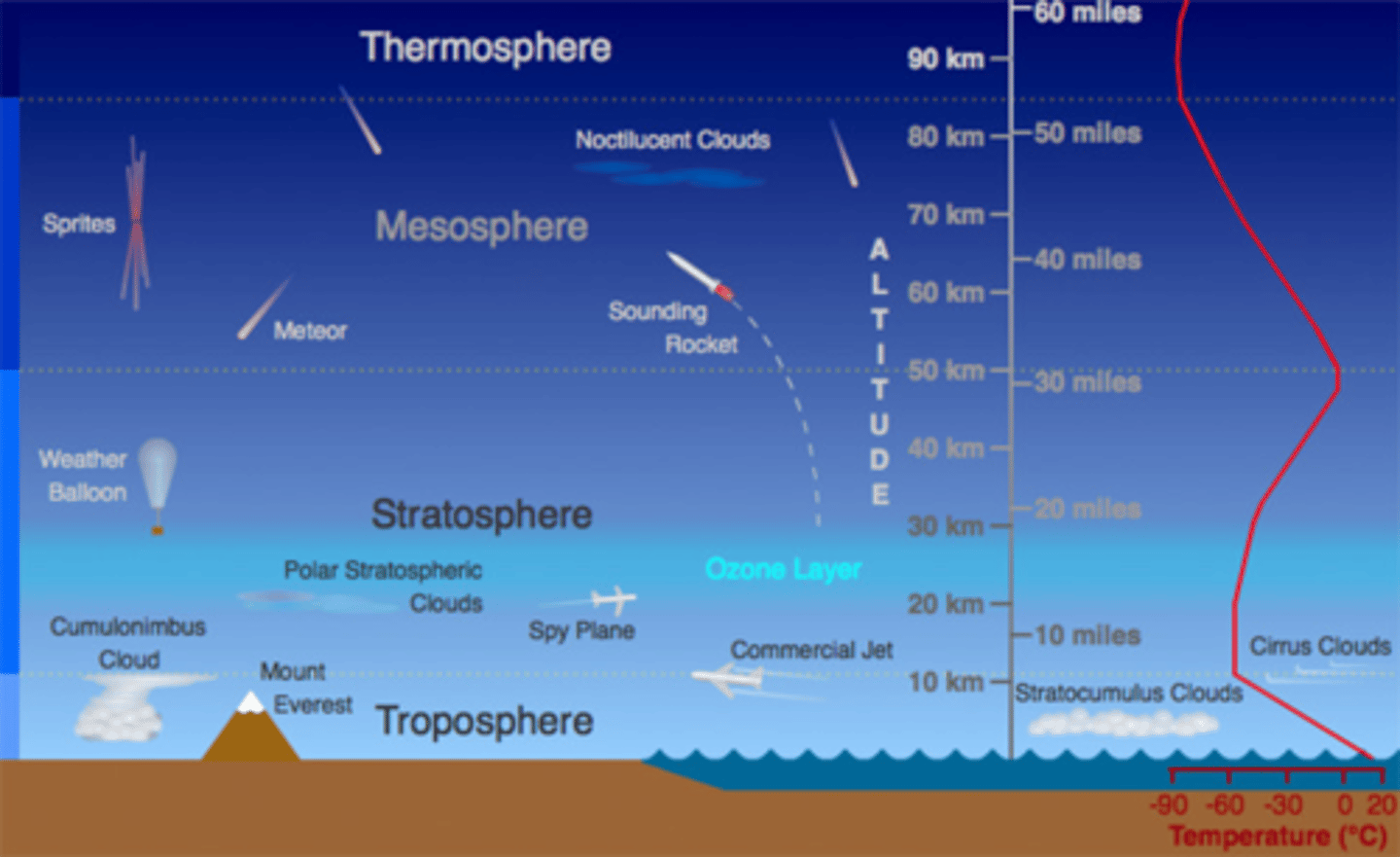
Name the layers of the atmosphere in order
Closest to Earth- Troposphere, Stratosphere, Mesosphere, Thermosphere
Define troposphere
The lowest layer of the Earth's atmosphere extending from the Earth's surface up to about 7km (above the poles) and about 20km (above the tropics)
Define stratosphere
The second layer of the Earth's atmosphere containing "ozone layer" about 10-50 km above the Earth's surface
What is the ionosphere?
An electrically charged layer of the Earth's upper atmosphere.
Includes the mesosphere and the thermosphere and extends up to 550 km?
Which are the two most chemically important regions of the atmosphere?
Troposphere and the stratosphere
Where are most molecules in the atmosphere found?
90% of all molecules in the atmosphere are in the troposphere
How does the density of the atmosphere change?
The atmosphere becomes less dense the higher you go
What two factors change with altitude?
Density- higher up is less dense
Temperature- changes with altitude
What does dry air mean?
No water vapour
List the average composition by volume of dry air from an unpolluted environment in the troposphere of the following gases:
Nitrogen (N2)
Oxygen (O2)
Argon (Ar)
Carbon Dioxide (CO2) *
Neon (Ne)
Helium (He)
Methane (CH4) *
Krypton (Kr)
Hydrogen (H2)
Dinitrogen Oxide (N2O) *
Carbon monoxide (CO)*
Xenon (Xe)
Nitrogen monoxide (NO) and nitrogen dioxide (NO2/ NOx)*
Nitrogen (N2)- 78%
Oxygen (O2)- 21%
Argon (Ar)- 1%
Carbon Dioxide (CO2) *- 399ppm
Neon (Ne)- 18.2ppm
Helium (He)-5.2ppm
Methane (CH4) *- 1.8ppm
Krypton (Kr)- 1.1ppm
Hydrogen (H2)- 0.5ppm
Dinitrogen Oxide (N2O) *- 0.3ppm
Carbon monoxide (CO)*- 0.1ppm
Xenon (Xe)- 0.09ppm
Nitrogen monoxide (NO) and nitrogen dioxide (NO2/ NOx)*- 0.003ppm
List the 5 gases in the atmosphere which gases are found naturally, but human activity increases their concentration
Carbon dioxide, methane, dinitrogen oxide, carbon monoxide and nitrogen monoxide/ nitrogen dioxide
What does human activity do to the atmosphere
Pollutes it by adding more gases
Some gases are already present in the atmosphere due to natural processes (CO2) but human activity increases their concentration
The main sources of some gases is a result of human activity
Describe how pollutants mix together
Over time gases mix together- natural diffusion process this is sped up in the atmosphere by air currents and prevailing winds meaning pollutant gases spread around the atmosphere leading to atmospheric pollution some of which depletes the ozone layer in the stratosphere
Describe how gases mix together in the troposphere
Mixing easily occurs in the troposphere as hot gases can rise and cold gases can fall.
Describe how gases mix together in the stratosphere
Mixing- Reverse temperature gradient- mixing is much more difficult in the vertical direction. But Horizontal circulation is rapid in the stratosphere especially around circles of latitude.
What can be done to try and limit the damaging effects of air pollution
International agreements made to reduce emissions
What is the main source as a result of human activity of carbon dioxide?
Combustion of hydrocarbon fuels (power stations/motor vehicles), deforestation
What is the main source as a result of human activity of methane?
Cattle farming, landfill sites, rice paddy fields, natural gas leakage
What is the main source as a result of human activity of nitrous oxide?
Fertilised soils, changes in land use (from the soil when land is ploughed up)
What is the main source as a result of human activity of carbon monoxide?
Incomplete combustion of hydrocarbons (from car exhausts)
What is the main source as a result of human activity of nitrogen oxide?
Internal combustion engines, from the reaction of N2 and O2 at high temperatures
Describe in 8 steps how the composition of the atmosphere has changed over time
1. The first atmosphere was lost during the upheavals in the early years of the solar system
2. The next atmosphere was made of carbon dioxide, methane and ammonia which bubbled out of the Earth
3. 3000 million years ago there was little oxygen in the atmosphere
4. First simple plants appeared and started to produce oxygen through photosynthesis
5. Over 1000 million years very little of this oxygen reached the atmosphere, used up quickly as it oxidised sulphur and iron compounds and other chemicals in the Earth’s crust
6. Once this was complete oxygen started to collect in the atmosphere
7. Oxygen concentrations reached 10% which was enough for the first animals to evolve using oxygen for respiration
8. Eventually there was enough respiration and other processes to remove the oxygen as fast as it was formed so since then the oxygen concentration has remained at around 21%
What are the two measures of gas concentration?
Percentage composition and parts per million
Describe percentage composition as a measure of gas volume and when it might be used
Means the parts per hundred - useful for abundant gases like nitrogen 78% of the troposphere
Percentage compositions e.g. 78% N- for every 100 particles in the atmosphere 78 of them are nitrogen
Describe parts per million as a measure of gas volume and when it might be used
ppm- useful for gases with a lower abundance/ small concentration e.g. argon is 0.09ppm which is 0.000009% and is easier to show in ppm as very small percentages are not easy to work with
E.g. 399ppm means in one million particles in a sample of air 399 of them will be carbon dioxide molecules
How do you convert percentage composition to parts per million?
Multiply by 10,000
How do you convert parts per million to percentage composition?
Divide by 10,000
1. Divide the number of molecules of a gas by 1 million e.g. 399/ 1000000= 3.99 x10-4
2. Multiply the answer by 100 to get the percentage= 3.99 x10 -4 x 100= 0.04%
This is the same as dividing 399 by 10,000
A concentration of 1% is the same as 10,000 ppm