elements, compounds and mixtures.
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
13 Terms
define element
substance that Is only made up of one type of atom
define compound
substance that is made up of two or more elements that are chemically combined
define mixture
Made up of two or more substances, they are not chemically combined.
in a pure substance what will the boiling and melting point be
at a fixed melting and boiling point
in a mixture what will be the boiling and melting point be
melting and boiling point can vary because they contain multiple substances.
what are the five ways that you can separate mixtures
simple distillation
fractional distillation
chromatography
filtration
crystallisation
what is simple distillation
simple distillation is used when a liquid is needed to be separated from a solution.
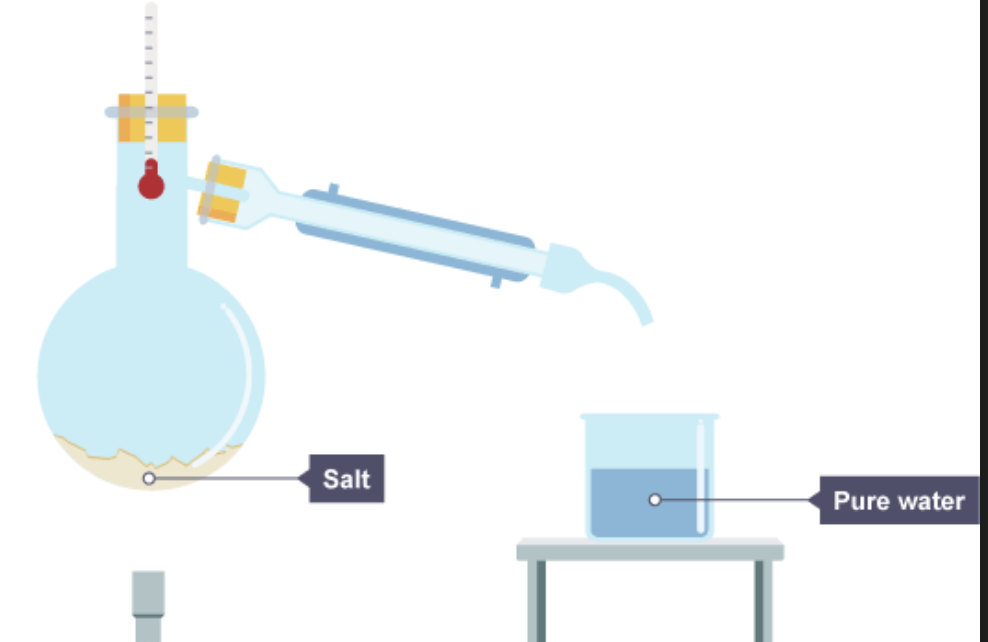
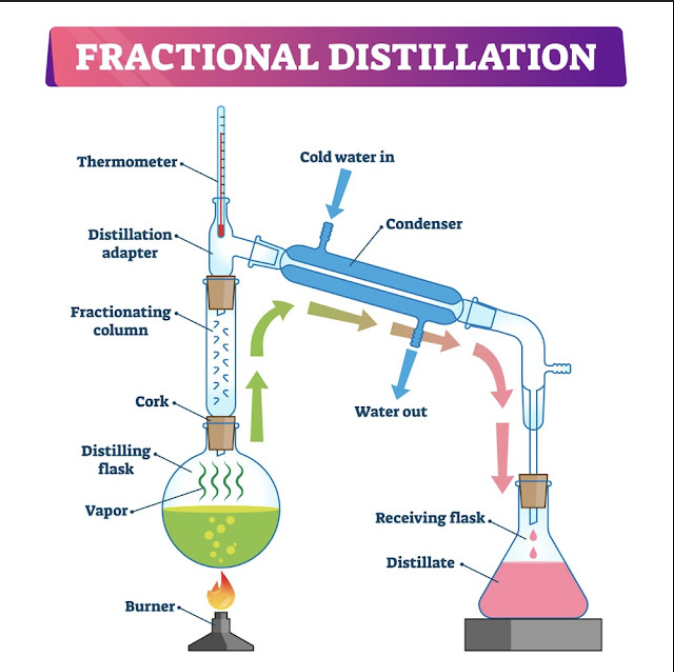
what is fractional distillation
fractional distillation is used when there is needed to separate multiple liquids from a mixture.
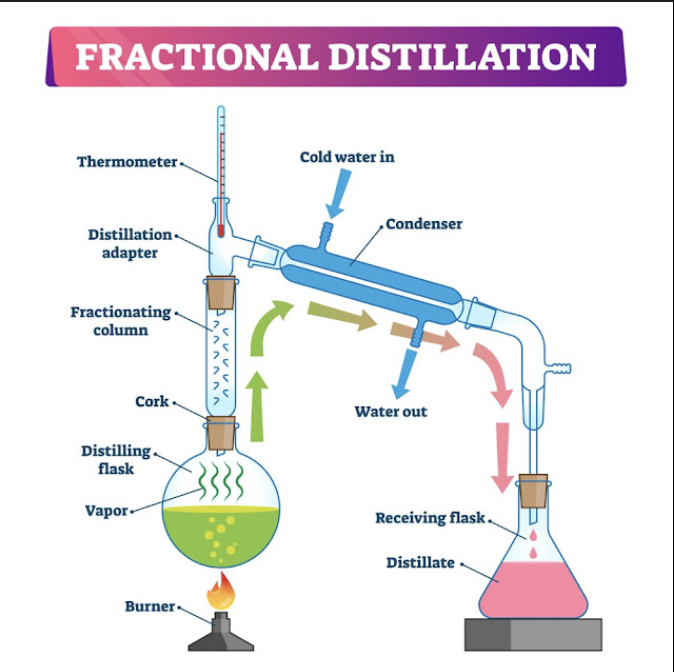
what is the difference that fractional distillation has than simple distillation?
fractional distillation has column rods, they are used for when the mixture is heated up multiple liquids that have similar boiling points will evaporate. Then column rods will condense the liquids except one liquid.
What is filtration
separates an insoluble solid from a liquid
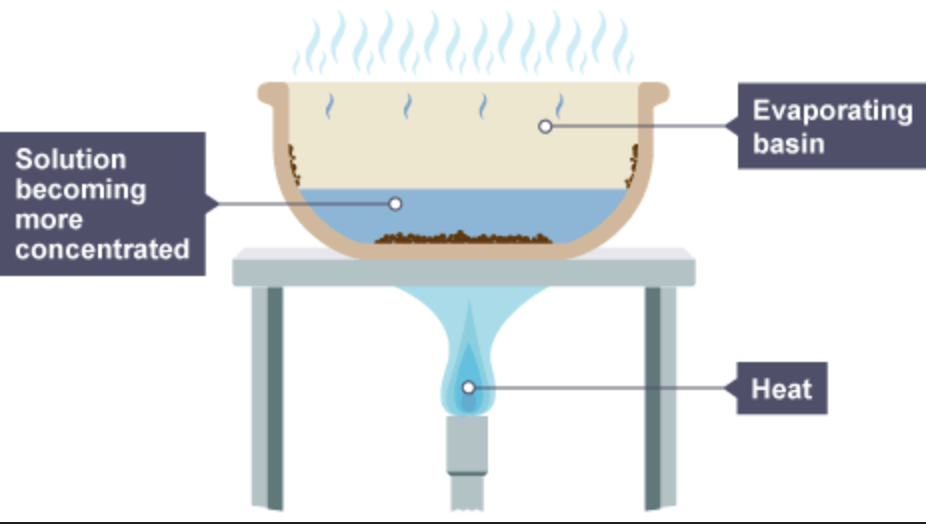
What is crystallisation?
separates solid from a solution by forming crystals
what is chromatography
separates substances based on solubility.
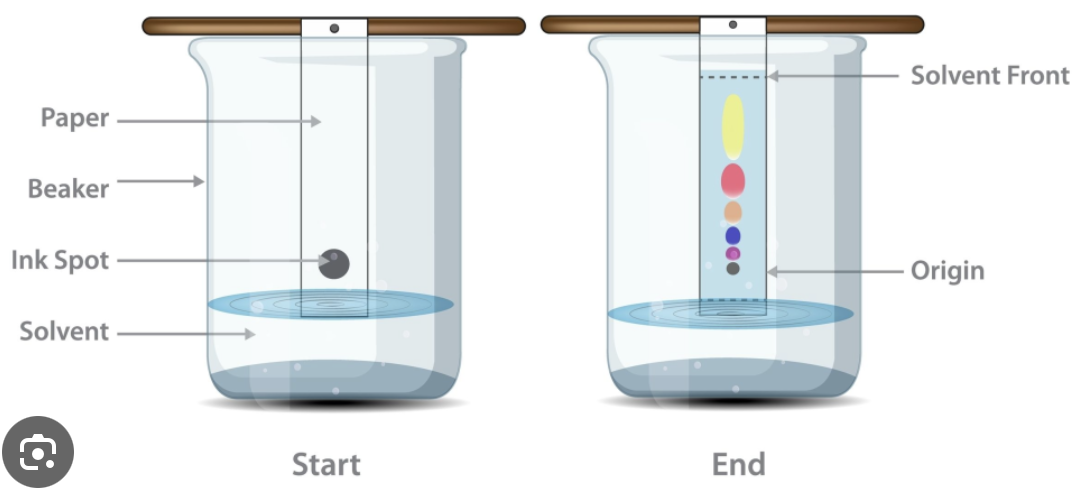
for the chromatography, how would you find the rf value?
distance moved by substance / distance moved by the solvent.