Corrosion
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
What is corrosion?
Gradual deterioration/destruction of metal/alloy starting at its surface by chemical or electrochemical reaction between the metal and the surrounding environment (air, moisture) is called corrosion. It can be a slow or fast process based on nature of metal and the surrounding environment.
Eg.: Rusting of iron: reddish brown deposition on iron and steel objects due to hydrated ferric oxide (Fe2O3.nH2O)
Eg.: Green scales on copper vessels due to the formation of basic cupric nitrate Cu(NO3)2 + Cu(OH)2
Why is the study of corrosion important for engineers and metallurgists?
Some consequences of corrosion:
severe economic loss
hampers safety of equipment
pipe leakages of inflammable and toxic gases can result in fire hazards and environmental pollution
failure of machine parts when metal becomes brittle
frequent replacement and maintenance of corroded parts → indirect financial loss → production loss
contamination of products (eg. traces of metals may alter the color of dyes; food articles in corroded containers may get spoilt)
25% of the annual production of iron is wasted because of corrosion
Why metals undergo corrosion?
Naturally, metals are present in their oxidized form in their ores.
During their extraction, lot of energy is put to reduce metal ions to metal atoms. Hence, metals are always in higher energy sate and thermodynamically unstable.
Metals try to lower their energy by spontaneously reacting with chemicals present in their surrounding environment like O2 and H2O.
This process of loss of metal is termed as metallic corrosion
Explain Electrochemical theory of corrosion by taking iron as example
When iron is exposed to atmosphere air O2 and moisture H2O the following changes occur:
→ Formation of minute anodic and cathodic areas on metal surface (galvanic cells)
At anodic area: Oxidation occurs
→ Fe oxidises to Fe2+ ferrous ions and liberates e-s
→ Metal is destroyed and lost at the surface due to dissolution
→ e-s released at anodic area move to the cathodic area through metal.
→ Fe2+ ions start moving towards the cathodic area through metal
At cathodic area:
→ Chemical species present on surface undergo reduction
→ The cathodic area provides the surface for reduction but remains unaffected
Depending on the environment, there are 3 possible reactions:
1) Air and moisture present (under neutral conditions)
½ O2 + H2O + 2e-s → 2OH-
2) Only moisture present and air absent (under neutral conditions)
2H2O + 2e-s → 2OH- + H2
3) Acidic medium in absence of air
2H+ + 2e-s → H2 ↑
→ The OH- ions formed move towards anode
Near the cathodic area:
Since → Fe2+ ions start moving towards the cathodic area through metal
→ The OH- ions formed move towards anode
→ Ferrous hydroxide is formed
Fe2+ + 2OH- → Fe(OH)2
On further reacting with Air and moisture we get hydrated ferric oxide
It is a brownish red corrosion product called rust
2Fe(OH)2 + ½ O2 + H2O → [Fe2O3 • 3H2O]
![<p>When iron is exposed to atmosphere air O<sub>2</sub> and moisture H<sub>2</sub>O the following changes occur:</p><p></p><p>→ Formation of minute anodic and cathodic areas on metal surface (galvanic cells)</p><p></p><p>At anodic area: Oxidation occurs</p><p>→ Fe oxidises to Fe<sup>2+</sup> ferrous ions and liberates e<sup>-</sup>s</p><p>→ Metal is destroyed and lost at the surface due to dissolution</p><p>→ e<sup>-</sup>s released at anodic area move to the cathodic area through metal.</p><p>→ Fe<sup>2+</sup> ions start moving towards the cathodic area through metal</p><p></p><p>At cathodic area:</p><p>→ Chemical species present on surface undergo reduction</p><p>→ <strong>The cathodic area provides the surface for reduction but remains unaffected</strong></p><p></p><p>Depending on the environment, there are 3 possible reactions:</p><p>1) Air and moisture present (under neutral conditions)</p><p><span style="color: red;"><strong><span>½ O</span><sub><span>2</span></sub><span> + H</span><sub><span>2</span></sub><span>O + 2e</span><sup><span>-</span></sup><span>s → 2OH</span><sup><span>-</span></sup></strong></span></p><p></p><p>2) Only moisture present and air absent (under neutral conditions)</p><p><span style="color: red;"><strong><span>2H</span><sub><span>2</span></sub><span>O + 2e</span><sup><span>-</span></sup><span>s → 2OH</span><sup><span>-</span></sup><span> + H</span><sub><span>2</span></sub></strong></span></p><p></p><p>3) Acidic medium in absence of air</p><p><span style="color: red;"><strong><span>2H</span><sup><span>+</span></sup><span> + 2e</span><sup><span>-</span></sup><span>s → H</span><sub><span>2</span></sub><span> ↑</span></strong></span></p><p></p><p>→ The OH<sup>-</sup> ions formed move towards anode </p><p></p><p>Near the cathodic area:</p><p>Since → Fe<sup>2+</sup> ions start moving towards the cathodic area through metal</p><p>→ The OH<sup>-</sup> ions formed move towards anode </p><p>→ <strong>Ferrous hydroxide</strong> is formed </p><p><span style="color: red;"><strong><span>Fe</span><sup><span>2+</span></sup><span> + 2OH</span><sup><span>-</span></sup><span> → Fe(OH)</span><sub><span>2</span></sub></strong></span></p><p></p><p>On further reacting with Air and moisture we get <strong>hydrated ferric oxide</strong></p><p>It is a brownish red corrosion product called rust</p><p></p><p><span style="color: red;"><strong><span>2Fe(OH)</span><sub><span>2</span></sub><span> + ½ O</span><sub><span>2</span></sub><span> + H</span><sub><span>2</span></sub><span>O → [Fe</span><sub><span>2</span></sub><span>O</span><sub><span>3</span></sub><span> • 3H</span><sub><span>2</span></sub><span>O]</span></strong></span></p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/404efe7c-55f5-4494-b289-6b74cfa38fbc.jpg)
What are the types of corrosion?
Differential Metal Corrosion (Galvanic or Bimetallic corrosion)
Differential Aeration corrosion (Concentration cell corrosion)
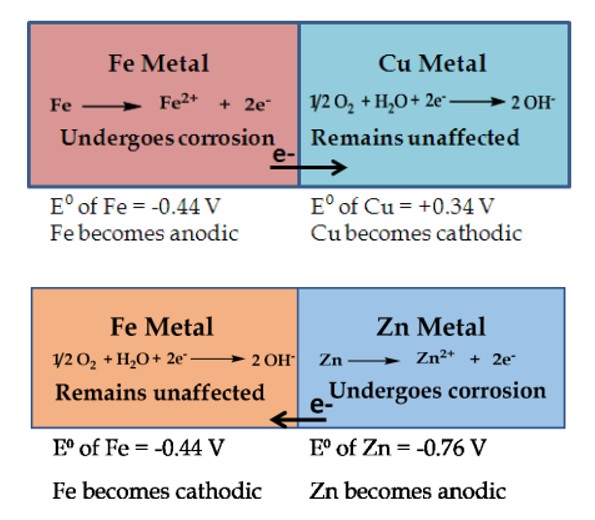
What is Differential Metal Corrosion
2 dissimilar metals are in contact with eachother in a corrosive environment
metal with lower electrode potential → undergoes oxidation → undergoes corrosion
metal with higher electrode potential → remains unaffected
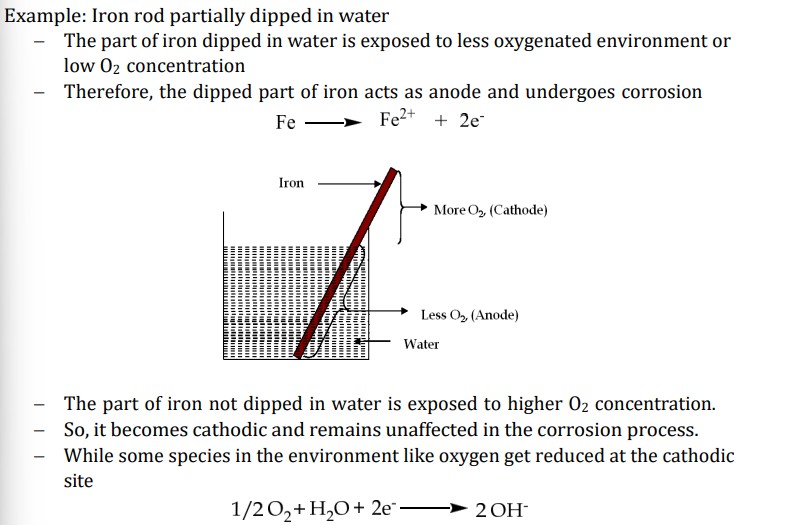
What is Differential Aeration Corrosion
occurs when metal is exposed to different concs of air (O2)
part of metal exposed to lower conc → lower electrode potential → anodic → undergo corrosion
part of metal exposed to higher conc → higher electrode potential → remain unaffected
eg. part of the nail inside the wall, window rods inside the frame, partially buried pipeline in soil, paper pins inside paper
What are the different types of Differential aeration corrosion
Water line corrosion
Pitting corrosion
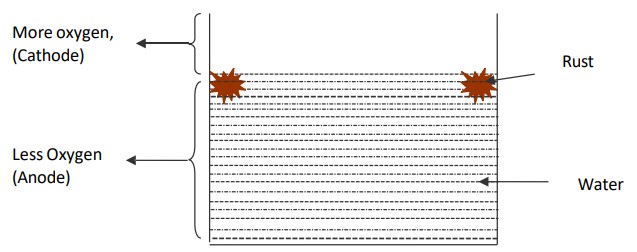
What is water line corrosion
Part of water tank below water line is exposed to low O2 conc.
eg. steel/iron tanks partially filled with water and ships floating in ocean for long time
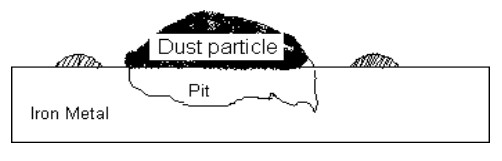
What is pitting corrosion?
observed when dust particles or oil drops get deposited over the surface of metal
portion of metal covered is less aerated (less oxygenated) → anodic → corrosion → forms deep narrow pit
area not covered → exposed to higher conc of O2 → cathodic → unaffected
Why is pitting corrosion more dangerous form of corrosion?
Pitting corrosion is a more dangerous, intense and localized form of corrosion.
Because, once pit is formed, the corrosion rate is accelerated due to further decrease in concentration of O2 within pit.
Hence, in a short duration, a narrow pit is formed resulting in the sudden breakdown of the metal.
What is stress corrosion?
occurs when some part of the metal is under stress and exposed to specific corrosive environment.
stressed part becomes more reactive (anodic).
when this portion is exposed to specific corrosive environment, chemicals get chemisorbed on these sites and initiates corrosion.
What is stress corrosion cracking?
Stress corrosion decreases ductility, increases brittleness and results in cracking of metal surface which is called stress corrosion cracking.
Explain caustic embrittlement in boilers
It is a form of stress corrosion in boilers operating at high pressure, at stressed regions of the boiler.
Specific corrosive environment: NaOH (caustic soda)
Boilers are made of mild steel
they undergo corrosion at the stressed portion when exposed to hot concentrated alkaline solution (NaOH)
Na2CO3 is added to reduce scaling.
When boiler feedwater is boiled: Na2CO3 undergoes hydrolysis to give NaOH.
Na2CO3 + H2O → 2NaOH + CO2
→ NaOH formed flows into hairline cracks by capillary action and accumalates.
When conc of NaOH>10% then it reacts with Fe to give Na2FeO2 (sodium ferrite)
Fe + 2NaOH → Na2FeO2 + H2
which decomposes into Fe3O4 (ferrosoferric oxide) and NaOH
3Na2FeO2 + 4H2O → Fe3O4 + 4H2 + 6NaOH
NaOH is replenished → Cyclic attack → Corrosion Cracking → Boiler failure
List all the factors affecting rate of corrosion
Nature of metal
Relative anodic and cathodic area
Nature of corrosion product
pH
Temperature
Conductance of medium
How does Nature of Metal influence corrosion rate?
→ metals with lower electrode potential like active metals (K, Na, Mg, Zn) → more reactive → corrode
→ metals with higher electrode potentials like noble metals (Ag, Au, Pt) → less susceptible
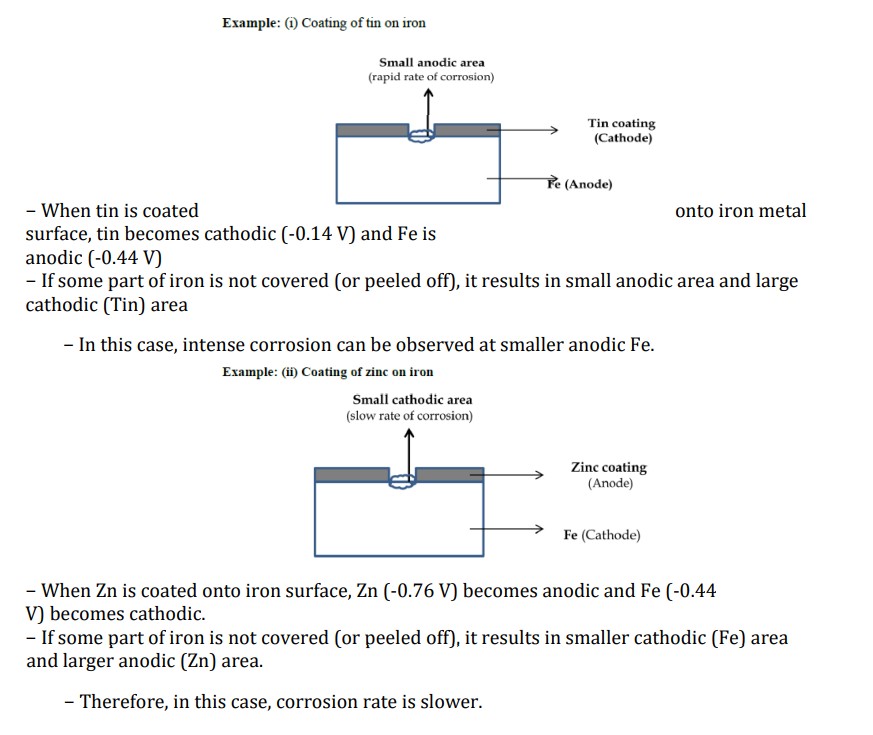
Why corrosion rate is faster when anodic area is smaller?
When metal corrodes, part of it becomes anode another part becomes cathode.
Larger cathodic area → Higher demand for consuming electrons → smaller anodic area tries to fulfil the demand of cathode by rapid liberation of electrons
How does nature of corrosion product affect corrosion rate?
→ may or may not prevent further corrosion
→ most metals form their oxides as corrosion products (thin film/layer)
→ if oxide is highly insoluble, stable, non-porous → protective
Eg.: The corrosion products of Al, Cr, Ti, etc. form passive oxide layers over the metal surface preventing further corrosion of metal
Eg.: The corrosion products of Fe, Zn, Mg, etc. unstable and therefore, do not prevent further corrosion of metal.
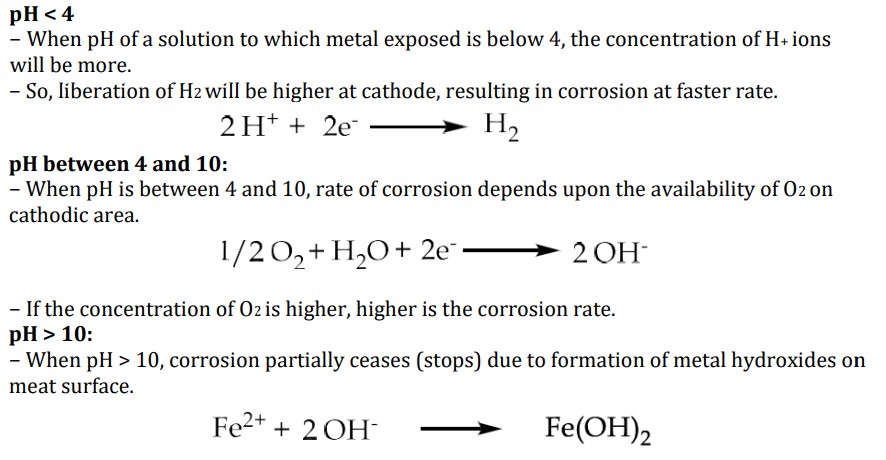
Explain how pH affects the rate of corrosion and the different cases
Corrosion rate increases with decrease in pH
pH of the solutions decides the type of cathodic reaction
Note: some metals like Al, Zn etc. undergo corrosion in alkaline medium in a faster rate.
How and why does temperature affect rate of corrosion?
Increase in temperature → Increase in conductance → decrease in polarization → increased corrosion rate
Example:
Rate of O2 reduction is doubled for every 30 °C rise in temperature
Rate of H2 evolution is doubled for every 10 °C rise in temperature
How does conductance of medium affect corrosion? Give example
Rate of corrosion increases with increase in the conductance of the medium
Higher the conductivity of medium → migration of ions between the anodic and cathodic regions is faster → decrease in polarization → increase in corrosion rate.
Eg. Metal immersed in sea water undergoes faster corrosion than the metals dipped in river water/normal water/distilled water. This is because sea water contains high concentration of salt, Na+ and Cl- ions.
List the methods commonly used to control corrosion
Protective coating
Cathodic protection
Anodic protection
Corrosion inhibitors
Design and selection of materials
What is cathodic protection?
The method of protecting a metal against corrosion by making the entire metal cathodic by providing electrons from external source.
→ Anodic area is eliminated by converting the entire metal into cathode.
Can be done done in 2 ways:
Sacrificial Anode method
Impressed Current method
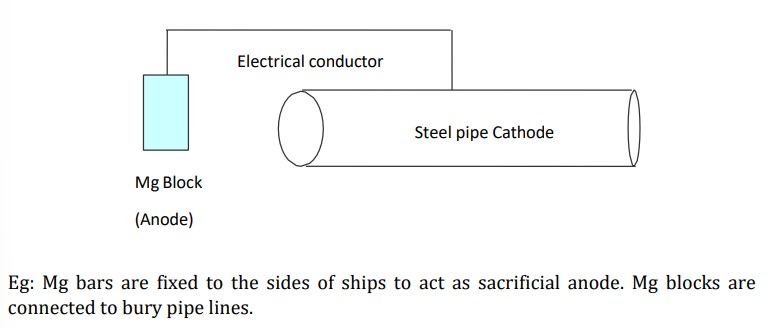
What is Sacrificial anode method?
→ metal to be protected is connected to more active metal
→ when in contact → anodic metal is anodic area and entire iron is cathodic area → galvanic cell is set up
→ anodic metal undergoes oxidation and provides e-s to iron
→ entire specimen becomes cathodic and protected
→ in the process, anodic metal undergoes corrosion by sacrificing itself hence they are frequently replaced
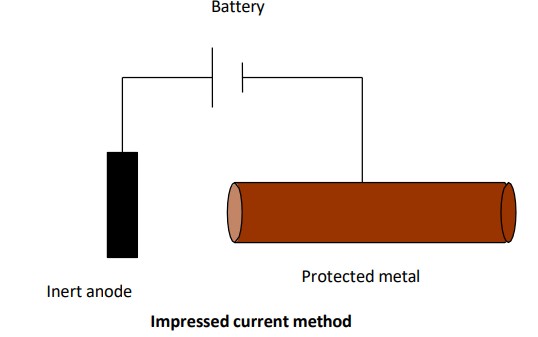
What is Impressed Current method?
→ e-s are supplied to the metal from external source
→ metal to be protected is connected to -ve terminal of DC source
→ +ve terminal to inert anode like graphite to complete the circuit
→ In such condition/set up, metal acts as cathode, does not undergo corrosion and remains unaffected.
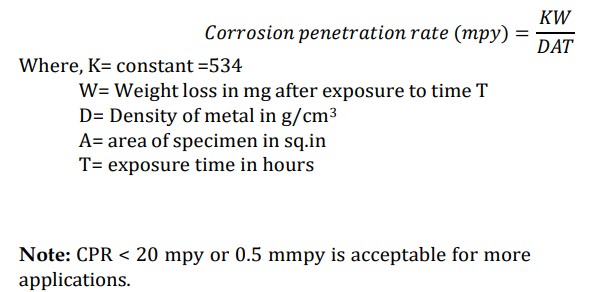
What is CPR
“Corrosion penetration rate (CPR) is defined as the speed at which any metal in a specific environment deteriorates due to a chemical reaction.
→ It indicates the amount of corrosion loss per year in thickness and the speed at which corrosion spreads to the inner portions of a material.
→ The rate of deterioration depends on the environmental conditions and the nature of metal under consideration.
expressed in Mils penetration per year (mpy) or millimetre per year (mmpy).
What are the general methods to measure corrosion rate?
1. Weight loss method: Weight loss of the metal before and after exposure to the corrosive environment.
2. Electrochemical methods: Measurement of corrosion potential or corrosion current. Corrosion tendency is measured by coupling the test metal with standard half-cell and determining the corrosion current or potential
Explain weight loss method
→ used for measurement of uniform corrosion
→ exposure of weighed peice of test metal/alloy to a specific environment for precise time
→ accompanied by thorough cleaning to remove corrosion products
→ determine weight of loss metal
The corrosion rate is best described in terms of the thickness or weight loss where the surface of the metal disintegrates uniformly across the area that has been exposed
CPR Formula
What is metal finishing?
Wide range of processes carried out in order to modify the surface properties
What is the technological importance of metal finishing?
✓ A decorative appearance.
✓ An improved corrosion resistance.
✓ An improved heat resistance.
✓ An improved surface hardness.
✓ An improved resistance to wear and tear.
✓ An improved resistance to abrasion.
✓ An improved solderability.
✓ Good thermal conductivity.
✓ Good electrical conductivity.
✓ Good thermal reflectivity.
✓ Good optical reflectivity
What is electroplating?
It is a process in which a layer of metal is deposited on the surface of another metal by applying electric current
The common metals used for electroplating are Zn, Cu, Ni, Cr, Ag, Pt, Au, etc.
Explain Electroplating of Gold
Plating bath used: Cyanide Baths (because cyanide is a complexing agent)
Depending on applications, plating is carried out in different pH range:
Acid cyanide bath:
→ pH range: 3.8 - 4.3
→ maintained using citric acid
→ used in gold plating for PCB, connnectors
Neutral cyanide bath:
→ pH: 6-8
→ monopatassium phosphate and potassium citrate
→ used for gold plating on semiconductors
Alkaline cyanide bath:
→ pH: 12
→ dipotassium phosphate and potassium cyanide
→ used for gold plating on semiconductors
What conditions are required for electroplating of gold on printed circuit boards and connectors?
ACID CYANIDE BATH (pH: 3.8-4.3)
Plating bath: Potassium gold cyanide and citric acid
Operating temp: 21-47°C
Anode: Platinum covered Niobium
Cathode: Object to be plated with gold
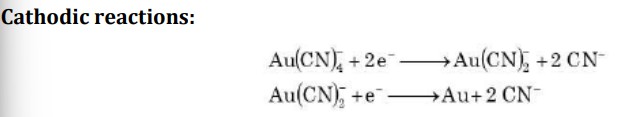
How is gold cyanide plating bath managed
→ Cyanide bath is unstable under highly acidic condition with pH < 3.5
→ Gold metal dissolves very fast in cyanides, therefore inert anode is used instead of pure gold metal
→ AuCl₃ is periodically added to cyanide bath to maintain the concentration of [Au(CN)₄]⁻.
→ Concentration of gold ions should be minimum in plating bath to obtain a good coherent deposit.
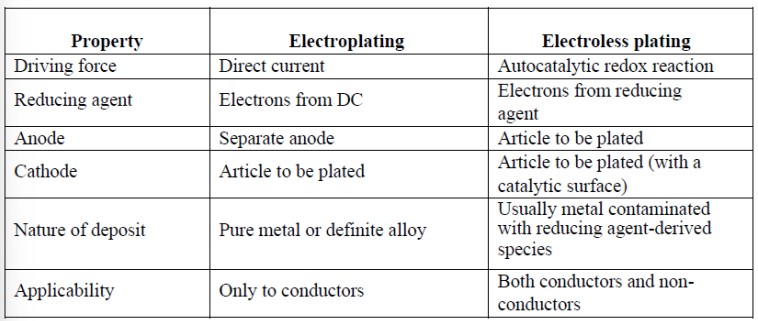
What is Electroless plating?
“Electroless plating is the method of depositing a metal or alloy over a substrate (conductor or non-conductor) by chemical reduction method without using electrical energy.
→ It is a non-galvanic plating method
→ It is a redox reaction.
→ It involves several simultaneous reactions in an aqueous solution, which occur without the use of external electrical power.
also known as Chemical plating or Autocatalytic plating

What are some advantages of Electroless plating over Electroplating?
→ Electrical energy is not used.
→ It helps in coating non-conducting, non-metallic surfaces like plastics, semiconductors, etc.
→ It has the ability to plate complicated and irregular shapes (edges, inside holes, etc).
→ It results in a coating with more consistent thickness.
→ Lower operating costs
→ Resulting deposits have unique physical, chemical, mechanical and magnetic properties
What are some disadvantages of Electroless plating over Electroplating
→ Rate of deposition is slow.
→ Analytical control of the bath is required.
→ Process could be expensive due to the usage of high-cost chemicals.
Difference between Electroplating and Electroless plating
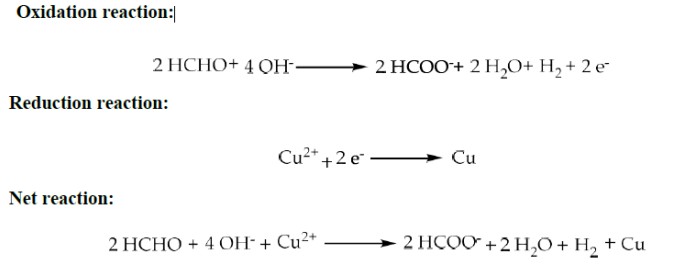
What are the steps in the method of Electroless plating of Copper?
In this method, Copper is deposited on a surface without passing electric current.
The surface is treated chemically to remove oils and other impurities like corrosive elements.
Surface activation is required
→ Etching by acid treatment.
→ The surface can be electroplated with a thin layer of metal to be plated, followed by heat treatment.
→ Insulators like plastics, glass, and circuit boards are activated chemically. The material is dipped in SnCl2 containing HCl at 25 °C, followed by PdCl2. SnCl2 reduces Pd2+ ions to Pd metal which is deposited on the surface of the material which catalyses redox reaction (coating).
Surface preparation is followed by dipping the metal to be coated in the plating bath.
Bath consists of;
→ Metal salt: 12 g/L solution of CuSO4
→ Reducing agent: 8g/L solution of HCHO (formaldehyde)
→ Buffer: 15 g/L NaOH + 55 g/L Rochelle salt
→ Complexing agent, 20 g/L EDTA
→ Stabilizer: Thiourea
→ pH: 11 – 12
→ Temperature: Room temperature
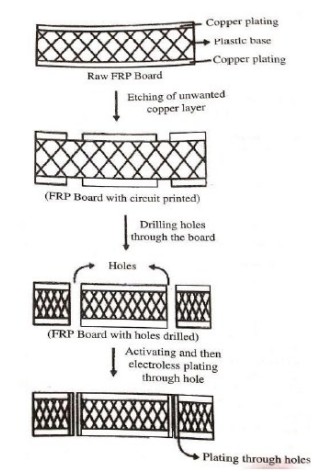
Electroless plating of Copper in Printed circuit boards
Electroless plating of Copper is used in the manufacturing of double sided, multi-layer PCBs. Through hole connections are necessary for the electrical connections between two sides of the board. It is done by drilling holes and then by electroless plating.
The steps involved are as follows:
Initially, the base object, plastic board is degreased and etched in acid.
The plastic board is then partially covered with copper foils (copper clad board).
The copper clad board is printed with the required pattern of the circuit.
On etching, copper below the printed pattern is etched away, leaving behind the circuit pattern.
The connection between 2 sides of PCB is made by drilling holes, activation (treating the board with SnCl2 containing HCl and PdCl2), followed by plating through holes by electroless plating.
The activated board is immersed in plating bath maintained at 25 ºC (room temperature).
Plating is carried out at a rate of 1 – 5 μm/h, till a thickness of 5 – 100 μm is obtained.
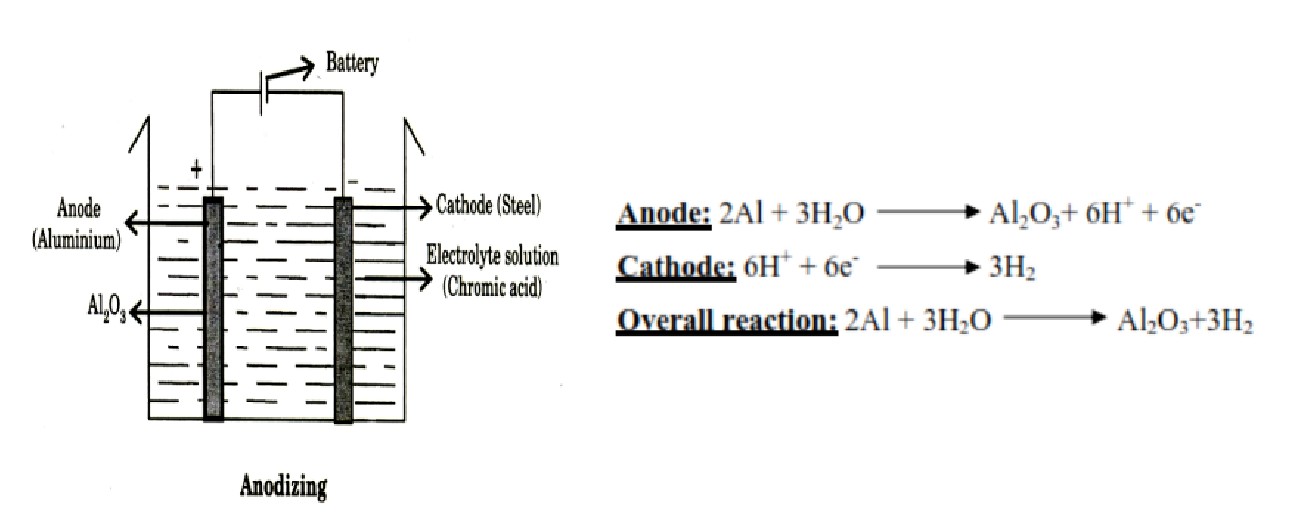
What is anodizing
Anodizing is a process of artificially forming protective passive oxide film on the surface of metals such as Al, Mg, Ti, Zr, Ta, Cr, etc by electrochemical oxidation.
Explain anodizing process
the article (aluminium) is cleaned, degreased, polished
taken as anode in an electrolytic cell
It is immersed in an electrolyte consisting of 5-10% chromic acid.
Steel or copper is taken as cathode.
Temperature of the bath is maintained at 35°C.
A current density of 100 Acm-2 or more (or applied potential 0-40 V) is applied which oxidizes outer layer of Al to Al2O3 that gets deposited over the metal.
The passivity of aluminium is due to the formation of a thin and compact layer of aluminium oxide over the metal. This phenomenon is known as anodizing or anodic oxidation or anodic passivity.
What are some uses of anodizing?
→ Anodized articles are used as an attractive, highly durable and corrosion resistant material
→in ceilings, floor, escalators, lobbies, staircase, curtain walls, tiffin carriers, soapboxes, household utensils, window frames, exterior for roofs, walls, buildings and in many home appliances.
→ Anodized aluminium is also used in computer hardware, exhibit displays, scientific instruments and in a range of home appliances and consumer products.
Difference between Surface Conversion Coating and Metal coating
Surface Conversion Coating: In this method outer surface of the base metal is converted into protective coating through chemical reactions. Example: Anodizing ,Phosphating
Metal Coating: Deposition of protective metal over the surface of base metal is known as metallic coatings. It is divided into anodic metal coatings and cathodic metal coatings.
What are anodic metal coatings
Anodic metal coatings are coatings which are anodic to the base metals.
Examples are aluminium, magnesium, zinc, and cadmium coatings on iron.
These metals are above iron in the galvanic series and undergo corrosion thereby protecting iron.
A characteristic feature of anodic coatings is that the base metal on which the coating is done will not get corroded even if the coating peals off.
This is due to the formation of large anodic and small cathodic areas.
Example: Galvanizing.
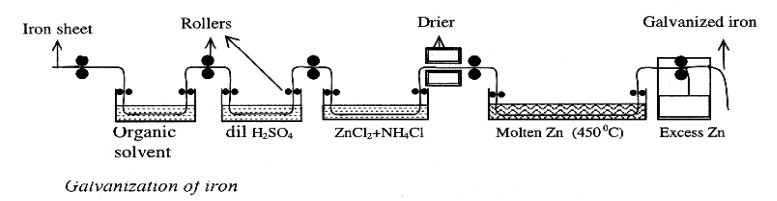
Explain Galvanizing Process for zinc coating on iron
The process of coating a layer of zinc on iron by hot dipping is called as galvanizing.
1) Iron sheet is passed through organic solvent to remove oil or grease present on it.
2) Then it is washed with dil.H2SO4 (pickling) to remove any rust (oxide layer) present on the surface
3) Then it is treated with a mixture of aqueous solution of zinc chloride (ZnCl2) and ammonium chloride (NH4Cl) which acts as flux and then dried.
4) The treated sheet is dried and dipped in molten zinc at 430-470 ºC.
5) Excess zinc present on iron sheet is removed by rolling, wiping or passing blast of air.
Uses of Galvanization
→ roofing sheets, buckets, bolts, nuts, nails, pipes etc.
→ Galvanizing and tinning by hot dipping is more economical than electroplating.
→ Hot dipping is limited to the coating of low melting point metals like Zn, Sn and Al over iron.