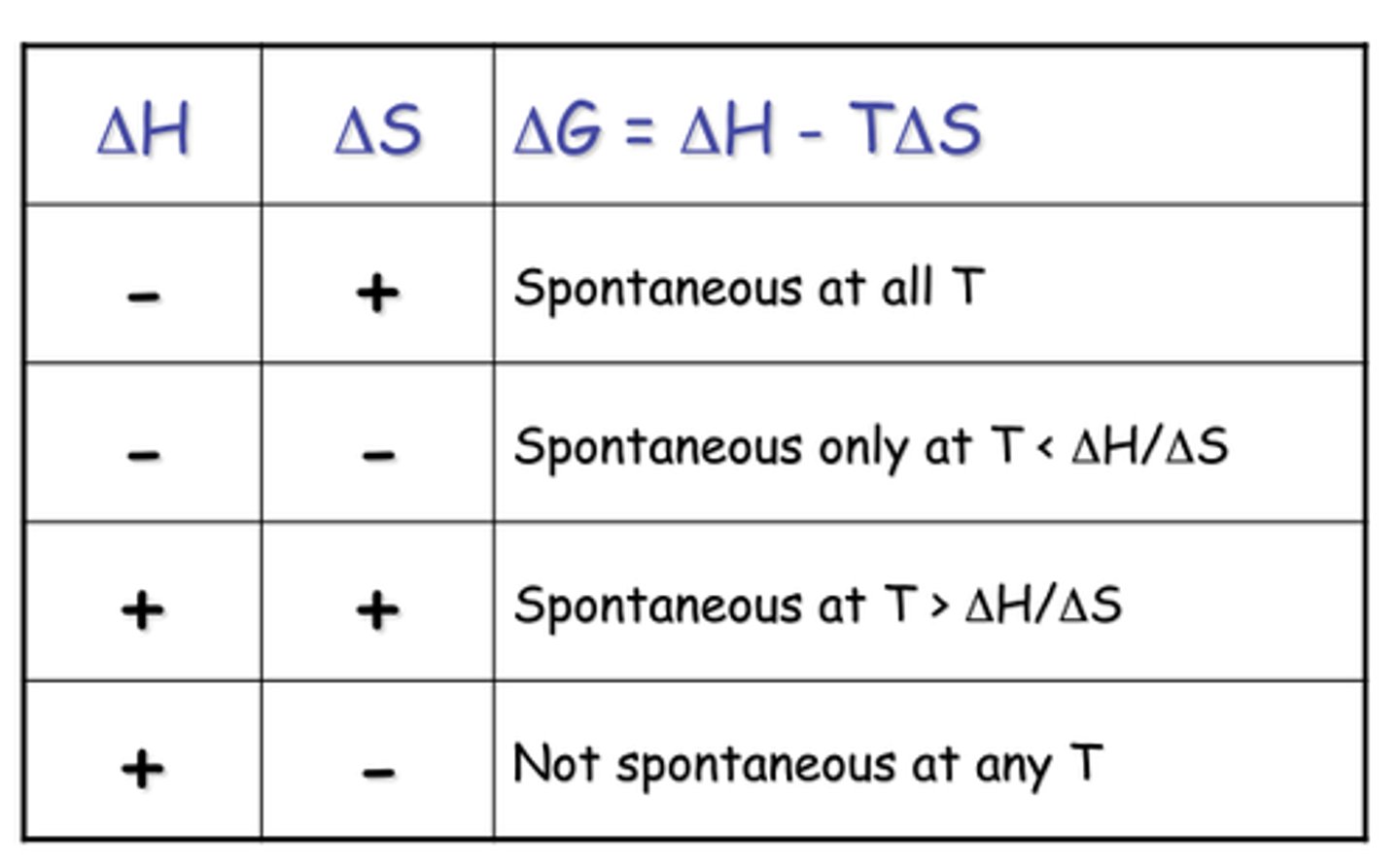
CHEM 1062 Ch. 5 Mastery Checkpoint
1/90
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
91 Terms
Why do we use Gibbs energy instead of considering the total entropy change when we are determining whether a process will occur?
Becaues Gibbs energy simplifies spontaneity predictions by focusing on system based variables at a constant temperature and pressure without calculating the entropy of the entire universe.

Imagine you have a mixture of ice and water. At a temperature of −10°C, what are the signs for ΔS, ΔH and ΔG for the process of water freezing?
ΔS: Negative(liquid-->solid, decreasing disorder)
ΔH: Negative(exothermic process, heat is being released to surroundings as liquid water transforms into solid ice)
ΔG: Negative(-10C is below freezing point so reaction is spontaneous)

Imagine you have a mixture of ice and water. At a temperature of +10°C, what are the signs for ΔS, ΔH, and ΔG for the process of water freezing?
ΔS: Negative(liquid-->solid, decreasing disorder)
ΔH: Negative(exothermic process, heat is being released to surroundings as liquid water transforms into solid ice)
ΔG: Positive(+10C is above the freezing point so reaction is nonspontaneous)

Imagine you have a mixture of ice and water. At a temperature of 0°C(273 K), what is the sign of ΔG?
ΔG: 0 (mixture of ice and water exists in equilibrium)

Your response correctly explains the significance of the sign of ΔG in determining whether a process is spontaneous, at equilibrium, or non-spontaneous.
ΔG indicates whether a reaction is spontaneous. Negative ΔG signifies a spontaneous reaction, and a positive ΔG signifies a nonspontaneous reaction; and 0 ΔG means the reaction is at equilibrium.
Explain why steam (gaseous water at 100 °C) will cause a more severe burn that liquid water at the same temperature. In your response, be sure to provide a molecular-level causal explanation.
Steam causes more severe burns because of the release of the latent heat of vaporization when the steam condenses on your skin, transferring a larger amount of thermal energy.

2NO2(g) ---> N2O4(g)
T= 298K
ΔS°= −175.8 J/K⋅mol
ΔH°=−57.2 kJ/mol
ΔG°=−4.8 kJ/mol
Is this reaction favorable at 298K?
Yes, becaues ΔG° is negative, meaning the reaction is spontaneous

2NO2(g) ---> N2O4(g)
ΔG°=−4.8 kJ/mol
What would you have to do to make this reaction "go further"? Justify your answer using the formula for ΔG.
Decrease the temperature, making ΔG° more negative. Temperature affects the spontaneity of a reaction. Increasing temperature if the reaction is endothermic or if it is exothermic, with an increase in entropy, or decreasing temperature if the reaction is exothermic with a decrease in entropy will make the reaction more spontaneous making it go further.
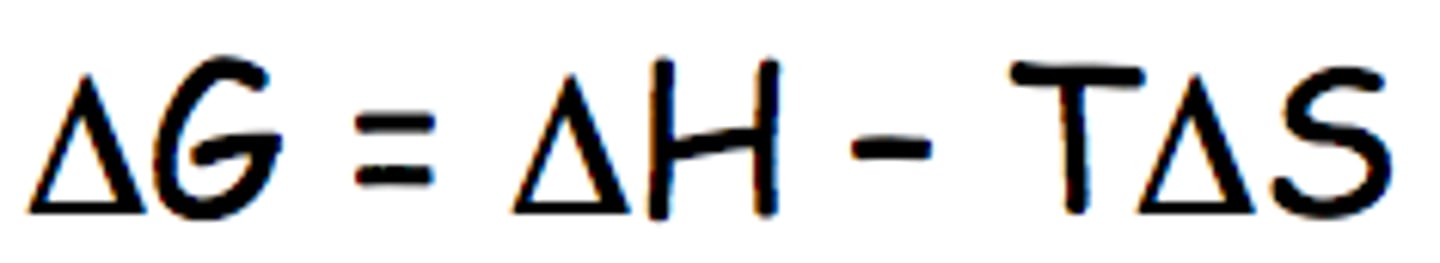
The rate in the forward direction is equal to the rate in the reverse direction.
Figure out the temperature at which equilibrium occurs since ΔG=0.
If ΔHvaporization of bromine (Br2) is 31 kJ/mol, and theΔS for vaporization of Br2 is 93 J/mol⋅K, what is the boiling point of Br2 in °C?
60.18°C
T=ΔH/ΔS
ΔHvap = 31 kJ/mol×1000J/1kJ=31000 J/mol
T= 31000 J/mol/ 93 J/mol*K= 333.33K
T= 333.33K-273.15= 60.18°C
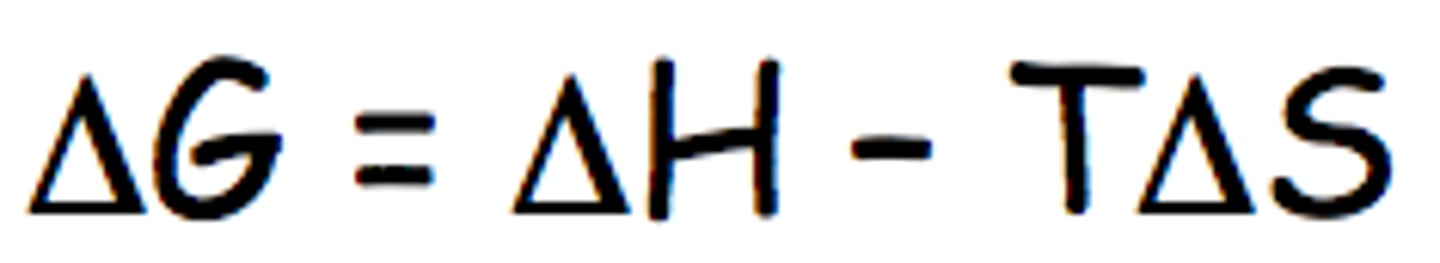
If ΔHvap (transitioning from liquid to gas phase) of water (H2O) is 40.7kJ/mol, and the ΔS for vaporization for H2Ois 109J/mol⋅K, what is ΔG for water vaporization at 350K? Does your answer make sense?
ΔG= 40.7kJ/mol - 350(109J/mol⋅K)
ΔG=407000-38159= 2550J/mol
The positive ΔG indicates that the reaction is nonspontaneous at 350K, which makes sense becaues it is below the boiling point for water, which is 373.15K, where vaporization is spontaneous.

C6H12O6(s)+6O2(g) ----> 6CO2(g)+6H2O(g)
What is the sign of ΔS, ΔH, ΔG
ΔS: +
ΔH: -
ΔG: -
Now let's look at the reverse of the combustion reaction: 6CO2(g)+6H2O(g) ----> C6H12O6(s)+6O2(g)
What is the sign of ΔS, ΔH, ΔG
ΔS: -
ΔH: +
ΔG: +
The ΔG for the overall reaction of photosynthesis is always positive, regardless of the temperature and is thus never favorable. However, we know this reaction happens every day. Explain how this reaction can happen even though ΔG is always positive.
Biological reactions aren't isolated, and unfavorable reactions can occur if they are coupled with favorable reactions. Captured energy (sunlight) drives the reaction, resulting in a favorable outcome.
Explain why we usually use ΔG instead of the total entropy change to predict whether a process is thermodynamically favorable.
ΔG is the measure of the system's free energy, allowing us to determine the spontaneity using the system variables. ΔStotal requires you to account for the entropy of the changes of both the surroundings and the system, which is often impractical to measure.
What is the sign of ΔGºrxn at equilibrium? Why?
0
Because there is no net change in the reaction's direction and the forward and reverse reactions occur at the same rate.
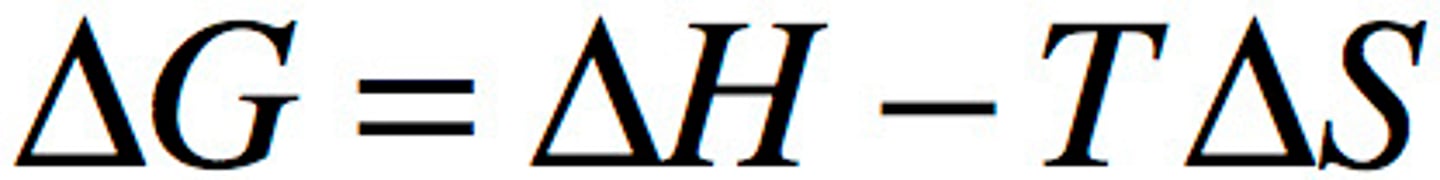
If for a given reaction, ΔH= 121.0 kJ/mol and ΔS = 25.4 JK−1 mol−1. Determine the value of ΔG (in kJ/mol) for the reaction at 25°C.
In what direction, if any, is the reaction spontaneous at this temperature?
T= 25+273.15= 298.15 K
ΔS= 25.4/1000= 0.0254 kJ K-1 mol-1
ΔG= 121.0 kJ/mol−(298.15 K×0.0254 kJ K-1 mol-1)
ΔG= 121.0-7.57351
ΔG= 113.43kJ/mol
The reaction is spontaneous in the other direction because ΔG is positive.
Using the table of thermodynamic data provided below, answer each of the questions listed below for the following reaction: 3A(g)+B(s)--->2C(g)+D(g). Find ΔSrxn∘, ΔHrxn∘ and ΔGrxn∘. Under standard conditions, in which direction would the reaction be spontaneous at 298 K?
ΔSrxn∘= -68.9
ΔHrxn∘= 138
ΔGrxn∘= 158.532
Reverse direction
Using the table of thermodynamic values provided here, determine ΔSrxn∘ at 298K for the conversion of ethylene gas into liquid benzene. 3C2H2(g) --->C6H6(l).
Based on your answer for part a) and using the fact that the enthalpy of the reaction, ΔHrxn= −631.1 kJ/mol what is ΔGrxn∘?
Under standard conditions, in which direction would the reaction be spontaneous at 298K?
ΔSrxn∘= -429.3
ΔGrxn∘= -503.1286
Forward direction because ΔGrxn is negative
If a reaction has ΔH = 103.0 kJ/mol, ΔS= 287.0 J K−1mol−1 determine the value of ΔG (in kJ/mol) for the reaction at 25∘C.
If the reaction is spontaneous at room temperature, at what temperature would it become non-spontaneous? If the reaction is non-spontaneous at room temperature, at what temperature would it become spontaneous? If the spontaneity of the reaction would not change, please enter 0 into the input field.
ΔGrxn∘= 17.474
T= ΔH/ΔS
T= 103*1000/287
T= 358.89 K
What is a spontaneous change?
A change that occurs under specific conditions without an ongoing input of external energy.
What is standard free energy change (ΔGº)?
The free energy change that occurs when all components of a system are in their standard state.
Which of the following correctly represents the relationship between ΔGº, ΔHº, and ΔSº?
ΔGº=ΔHº-TΔSº
Based on the information provided below, determine the standard free energy of formation, ΔGf∘, of H3PO4(l). Note that the ΔG∘ values for these reactions have been modified from their actual values so the answer cannot simply be found in the table of thermodynamic data.
You're combining the three given reactions to make the formation reaction for H₃PO₄. It's easiest to form 4 moles of H₃PO₄ and then divide by 4. (balance reaction)
Target (4 mol):
6 H₂(g) + P₄(s) + 8 O₂(g) → 4 H₃PO₄(l)
Obtain this by summing:
1. P₄ + 5 O₂ → P₄O₁₀ ; ΔG° = −2633.00 kJ
2. (×3) 6 H₂ + 3 O₂ → 6 H₂O ; ΔG° = 3(−448.12) = −1344.36 kJ
3. 6 H₂O + P₄O₁₀ → 4 H₃PO₄ ; ΔG° = −413.87 kJ
Sum ΔG° = −2633.00 + (−1344.36) + (−413.87)= −3977.36 + (−413.87)
= −4391.23 kJ (for formation of 4 mol H₃PO₄)
Per mole H₃PO₄: ΔGf° = (−4391.23 kJ) / 4 = −1097.8075 kJ/mol
A+B ---> C, The ΔGºf for A is 330 kJ/mol. The ΔGºf for B is 485 kJ/mol. The ΔGºf for C is 502 kJ/mol. What is ΔGºrxn? If both ΔH and ΔS are negative, which component makes it spontaneous, and in which direction is the reaction spontaneous?
ΔGºrxn= -313
Enthalpy; driven to the right. Because the favorable negative ΔH (exothermic reaction) outweighs the unfavorable negative ΔS (decreased disorder).
Shown below are two different ways to synthesize hydrogen peroxide, H2O2. Calculate ΔGrxn∘ for each of the reactions using the ΔGf∘ values provided. HX2(g)+OX2(g)--->H2O2(l) and H2O(l)+1/2O2(g)--->H2O2(l)
Of these two reactions, which is MORE spontaneous under these conditions?
Reaction A:
HX2(g)+OX2(g)--->H2O2(l)
ΔGrxn∘= -120.35
Reaction B:
H2O(l)+1/2O2(g)--->H2O2(l)
ΔGrxn∘= 116.76
Reaction A becaues it has a negative ΔG.
If ΔHrxn∘= -44.4kJ/mol and ΔSrxn∘= -168JK-1mol-1 for a hypothetical reaction, answer each of the questions shown below.
The reaction changes spontaneity at a temperature of:
The reaction is spontaneous at all temperatures ________ the answer in part a).
T=ΔH/ΔS
convert ΔH to jules(x1000)
264K
Below
A theoretical compound has a ΔHvap= 49.2049.20 kJ/mol and a ΔSvap= 71.50 J K−1mol−1. Determine the boiling point for this compound.
T=ΔH/ΔS
convert ΔH to jules(x1000)
T= 49200/71.50=
688.11 K
Explain the role of probability in entropy changes.
Probability directly controls changes in entropy because a system tends to go toward states that are statistically more probable, which increases the number of possible microstates and thus increases entropy. The number of possible microstates is a direct measure of entropy because a system naturally tends to move toward the most probable state. A macrostate with the highest number of possible microstates is the most statistically probable and corresponds to the highest entropy.
Predict the sign of the entropy change for each process:
H2(g) + Br2(l) ⇌ 2 HBr(g)
CaCO3(s) ⇌ CaO(s) + CO2(g)
2 Na(s) + Cl2(g) ⇌ 2 NaCl(s)
H2(g) + Br2(l) ⇌ 2 HBr(g): +, there is one mole of reactant gas, and there are two moles of product gas. We're going from a gas and a liquid to just gaseous products
CaCO3(s) ⇌ CaO(s) + CO2(g): +, there is one mole of reactant, and there are two moles of products. We're going from a solid to a solid and a gas.
2 Na(s) + Cl2(g) ⇌ 2 NaCl(s): -, Entropy is decreasing because we're going from a solid and a gas to a solid.
The second law of thermodynamics states that for any change, the total entropy of the universe must increase. Which of the following statements correctly identifies the relationship between the entropy of the universe (ΔSuniv), the entropy of the system (ΔSsys), and the entropy of the surroundings (ΔSsurr)?
ΔSuniv= ΔSsurr+ΔSsys
A law stating that a process occurs spontaneously in the direction that increases the entropy of the universe is the...
Second law of thermodynamics
What is energy dispersal?
The distribution of energy and opportunities from freedom of motion. Causes a decrease in systems ability to do work(ΔG)
Which of these is standard molar entropy?
The entropy of 1 mol of a substance in its standard state
What are the conditions of standard molar entropy for a gas? What are the conditions of standard molar entropy for a solution?
1 atm
1M
Determine how the entropy would change in each of the following processes:
He(l)→He(g)
CO2(g)→CO2(s)
Al(s,−15∘C)→Al(s,−196∘C)
He(l)→He(g): Increase
CO2(g)→CO2(s): Decrease
Al(s,−15∘C)→Al(s,−196∘C): Decrease because the system loses thermal energy as it cools, which reduces the random motion and possible arrangements of the aluminum atoms
Sort each the following compounds based on their standard molar entropy values, S∘ from largest to smallest. H2O(l), O2(g), N2(g), and CH3CH2OH(g)
CH3CH2OH(g)
O2(g)
N2(g) -more stable then O2
H20(l)
Sort each the following compounds based on their standard molar entropy values, S∘ from largest to smallest. HCI(g), H2(g), and HClO3(g)
HClO3(g)
HCI(g)
H2(g)
Sort each the following compounds based on their standard molar entropy values, S∘ from largest to smallest. O(g), O2(g), and O(3)g
O(3)g
O2(g)
O(g)
Sort each the following compounds based on their standard molar entropy values, S∘ from largest to smallest. Li(s), S8(s), P4(s)
S8(s)
P4(s)
Li(s)
Assuming you have equal amounts of each of the substances at the same temperature, rank each of the compounds in the following set from the highest down to the lowest absolute entropy. N2(l), NO2(g), O2(g), NO(g)
NO2(g)
NO(g)
O2(g)
N2(l)
Predict the sign of the entropy change, ΔS. Na+(aq)+Cl−(aq)→NaCl(s)
ΔS is negative
Predict the sign of the entropy change, ΔS. 2Fe(s)+3/2O2(g)→Fe2O3(s)
ΔS is negative
Predict the sign of the entropy change, ΔS. 2C6H14(l)+19O2(g) ---> 12CO2(g)+14H2O(g)
ΔS is positive
Determine which of the following reactions would have a positive ΔS.
2HBr(g)→ H2(g)+Br2(l)
P4O10(s)+6 H2O(l)→4 H3PO4(s)
2SO3(g)→2SO2(g)+O2(g)
2SO3(g)→ 2SO2(g)+O2(g)
Determine the change in standard entropy, ΔS∘, for the two reactions shown below using the thermodynamic data provided.
2CO(g)+O2(g)→2CO2(g)
3H2(g)+N2(g)→2NH3(g)
2CO(g)+O2(g)→2CO2(g) ΔS= -173.0
3H2(g)+N2(g)→2NH3(g) ΔS= -198.1
The ΔS of the system is 540 J/mol⋅K, and the ΔS of the surroundings is 245 J/mol⋅K. Will this process occur spontaneously?
Yes, this process will occur spontaneously because the ΔSS of the universe is positive overall. ΔSuniv= ΔSsurr+ΔSsys
Describe how heat entering a system impacts the entropy of the surroundings.
When heat transfers into a system, heat leaves the surroundings, causing a decrease in the temperature of the surroundings. This loss of heat energy from the surroundings leads to a decrease in the random motion and available configurations of the surrounding molecules, thus decreasing the entropy of the surroundings.
Determine ΔHrxn of the given reaction.
4NH3(g) + 5O2(g) ⟶4NO(g) + 6H2O(g)
NO(g): +90.29ΔHf
NH3(g): -45.9ΔHf
H2O(g): -241.82ΔHf
ΔHrxn= -906.74 kJ/mol
What are some examples of endothermic processes?
Vaporizing liquid propanol, melting solid iron, melting ice
What are some examples of exothermic processes?
Condensation of rain from water vapor, Deposition of CO2 gas into dry ice(solid CO2)
Freezing liquid water into snow
Burning log

What is a exothermic process?
A process that releases energy into its surroundings. ΔH-

What is an endothermic process?
A process that absorbs heat from the surroundings. ΔH+
What is ΔS
Change in entropy
What is ΔH
Change in enthalpy
A negative ΔH indicates an exothermic reaction (heat is released to the surroundings).
Apositive ΔH signifies an endothermic reaction (heat is absorbed from the surroundings).
What is ΔG
Gibbs free energy, indicates the spontaneity of a process under constant temperature and pressure. Measures tge energy available to do work.
What is entropy (S)?
Measure of disorder or randomness in a system.
2nd law of thermodynamic: For spontaneous processes ΔSuniv >0
ΔSuniv= ΔSsurr+ΔSsys
the total entropy of the universe (or an isolated system) always increases.
What is enthalpy(H)?
1st law of thermodynamic: energy can be created or destroyed.
The total heat content of a system at constant pressure. It's the sum of the system's internal energy and the energy it takes to make room for itself in its environment,
What does increase molecular mass do to entropy?
increase molecular mass = increase entropy
He
A spontaneous process will always__________total ΔSuniv
increase
Describe how heat leaving a system impacts the S of the surroundings.
When heat leaves the system, it goes into the surroundings and increases thermal energy and S of the surroundings.

What is spontaneous change?
a reaction occurs on its own without external input of energy.
More complex substances have _________ ΔS because there are more ways to arrange their atoms.
Higher
For the process of going from a liquid to a gas what are the signs of ΔHsys, ΔSsys, ΔG?
ΔHsys: + energy must be absorbed
ΔSsys: + increasing disorder
ΔG: Depends on temp., to make process go temp. needs to be increased.
What do you predict the sign for ΔSsys? 2NO2(g)--->N2O4(g)
Negative(-), because 2 moles of gas combine to form a single mol of gas.
What is ΔG at 295K and ΔS= -175.8 and ΔH=-52.2? 2NO2(g)--->N2O4(g)
Is this reaction favorable at 298K? What needs to happen to make the reaction go farther? What if we heated it up beyond equilibrium temp?
ΔG=-4.8
Yes because ΔG remains negative(-)
Cool it down because ΔG needs to remain negative(-).
ΔG would be + and the reaction would go into the reverse direction.
For water at 100C(373K) what is it doing? ΔHvap=40.65 what is ΔSvap?
ΔS=ΔH/T
ΔSvap=109
Products are _________stable because they have lower energy
more
What are the diatomic elements?
Hydrogen, Nitrogen, Oxygen, Fluorine, Chlorine, Bromine, and Iodine
Most elements are solid in their standard state. Which ones are liquid or gas in their standard state?
Liquid: Br2 & Hg
Gas: Hydrogen (H₂), Nitrogen (N₂), Oxygen (O₂), Fluorine (F₂), Chlorine (Cl₂), and the noble gases (Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn)
Write the ΔHf equation for NO(g) and calculate it.
1/2N2(g) + 1/2 O2(g) ---> NO(g)
114.1= [2x + 0] -2(33.2)
x=90.25
Draw on entropy diagram for 2H2(g)+O--->2H2O(g)
I Reactants
H^I 2H2(g)+O2(g)
I I
I I exothermic energy is released
I 2H2O I
Product '''
The spontaneity of a reaction depends on both enthalpy and entropy changes. Many reactions occur even if enthalpy and entropy changes(one or the other) of the system are not factorable. How can this be explained?
1. Reactions can be coupled, ΔG+ and ΔG- so ΔG is overall (-)
2. Even if ΔH is +, at increased temp. the process can occur(if ΔS is -)
3. At low temp ΔS- can overcome (if ΔH-)
4.Process can still happen with -ΔSsys if ΔSsurr is + and longer that ΔS and larger that ΔSsys.
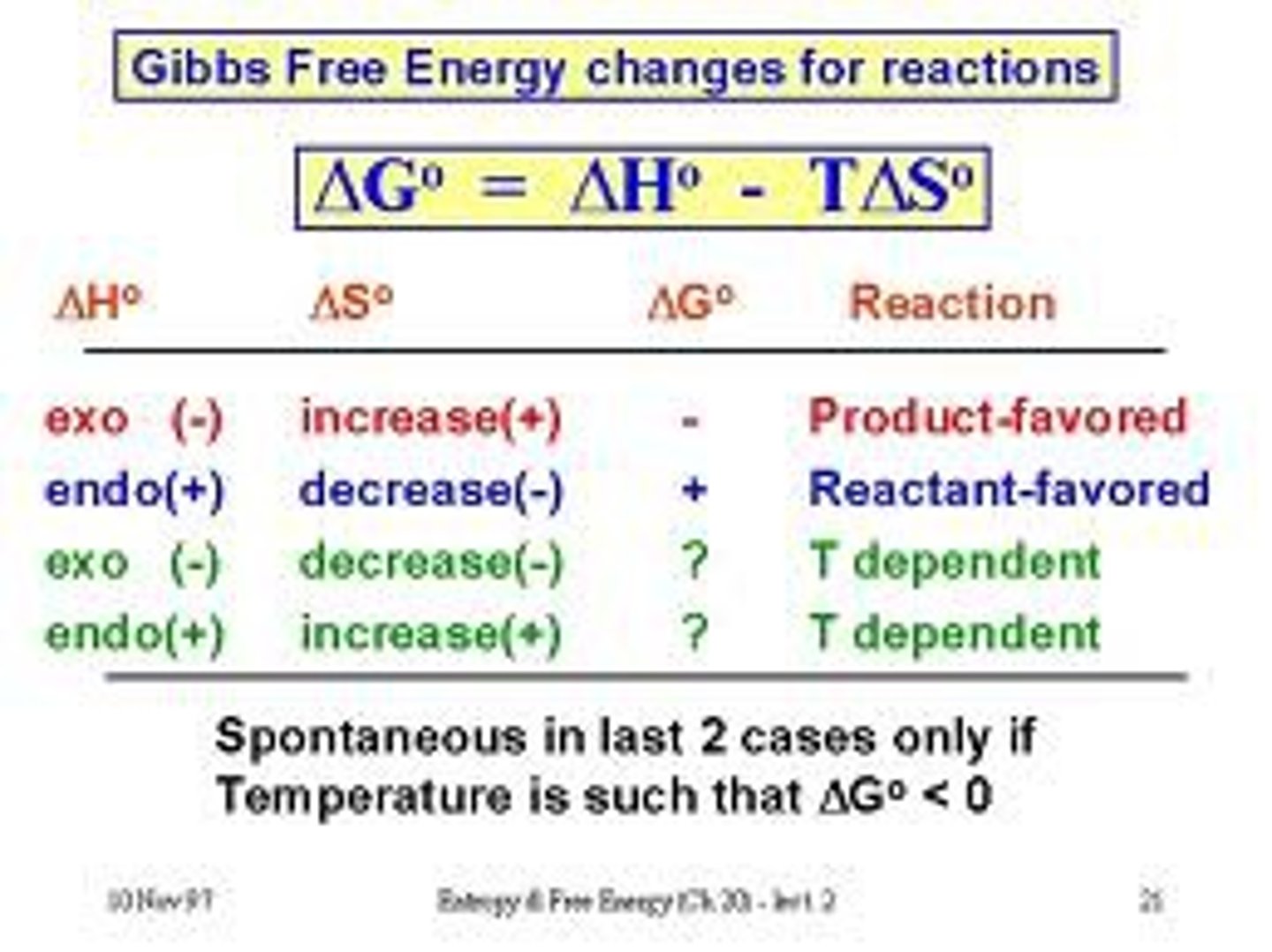
Consider a generic phase change, represented below. Will this phase change be accompanied by a temperature change of the system? Indicate your answer as increase, decrease, or no change. X(s) ⟶X(l)
There will be no change in temp during the phase transition because phase transitions proceed at a constant temp.
Determine the amount of heat energy (in kJ) added to heat a 57.4 g sample of ethanol (C2H5OH) from a liquid at 78.5°C to 95°C. Your answer will be a positive number.
q=mcΔT
c=1.70 J/g
q=50.35
The temperature at which a phase change occurs depends upon _____________.
the molecular structure of the compound
For molecular substances, as a substance changes, phase intermolecular forces are____________(not chemical bonds).
overcome
The direction of change is determined by_______________________or________________.
an increase in the total entropy change (ΔStotal) or the Gibbs energy change (ΔG)
Measuring temperature changes can be related to_________________________.
Molecular level changes in interaction strength by the thermodynamic function ΔH and bond energies.
Explain the difference/relationship between temperature, thermal energy, and kinetic energy.
Temperature measures the average kinetic energy of a substance's particles, while thermal energy is the total kinetic energy of all particles within that substance. Kinetic energy is the energy of motion itself, and in this context, it refers to the random movement of atoms and molecules.
Temperature measures the average kinetic energy of a substance's particles, while thermal energy is the total kinetic energy of all particles within that substance. Kinetic energy is the energy of motion itself, and in this context, it refers to the random movement of atoms and molecules.
This kinetic energy can be expressed as translational (movement through space), rotational (spinning), or vibrational (back-and-forth motion) energy
Explain why particles in gases move with a range of different velocities at a given temperature. Identify the Boltzmann distributions of particles at different temperatures or for particles of different molecular weights at a specified temperature.
Particles in a gas move with a range of different velocities due to random, elastic collisions that constantly redistribute kinetic energy among them, leading to a distribution of speeds rather than a single speed. The Maxwell-Boltzmann distribution describes these speeds, showing that at higher temperatures, the average speed and the spread of speeds increase, while for heavier particles at a constant temperature, the average speed and the distribution of speeds decrease, with the distribution shifting to lower velocities.
Explain the causes of water's anomalous properties (high melting point, boiling point, lower density of ice relative to liquid water, specific heat).
strong hydrogen bonds between water molecules. These bonds require significant energy to break,
Explain why the heat capacity of a substance is affected by the molecular-level structure.
The molecular-level structure affects a substance's heat capacity because it determines the number of "degrees of freedom" a molecule has for storing added energy. More complex molecules or those with stronger intermolecular forces have more ways to store energy (more degrees of freedom), requiring more total energy to increase their temperature, thus exhibiting higher heat capacities.
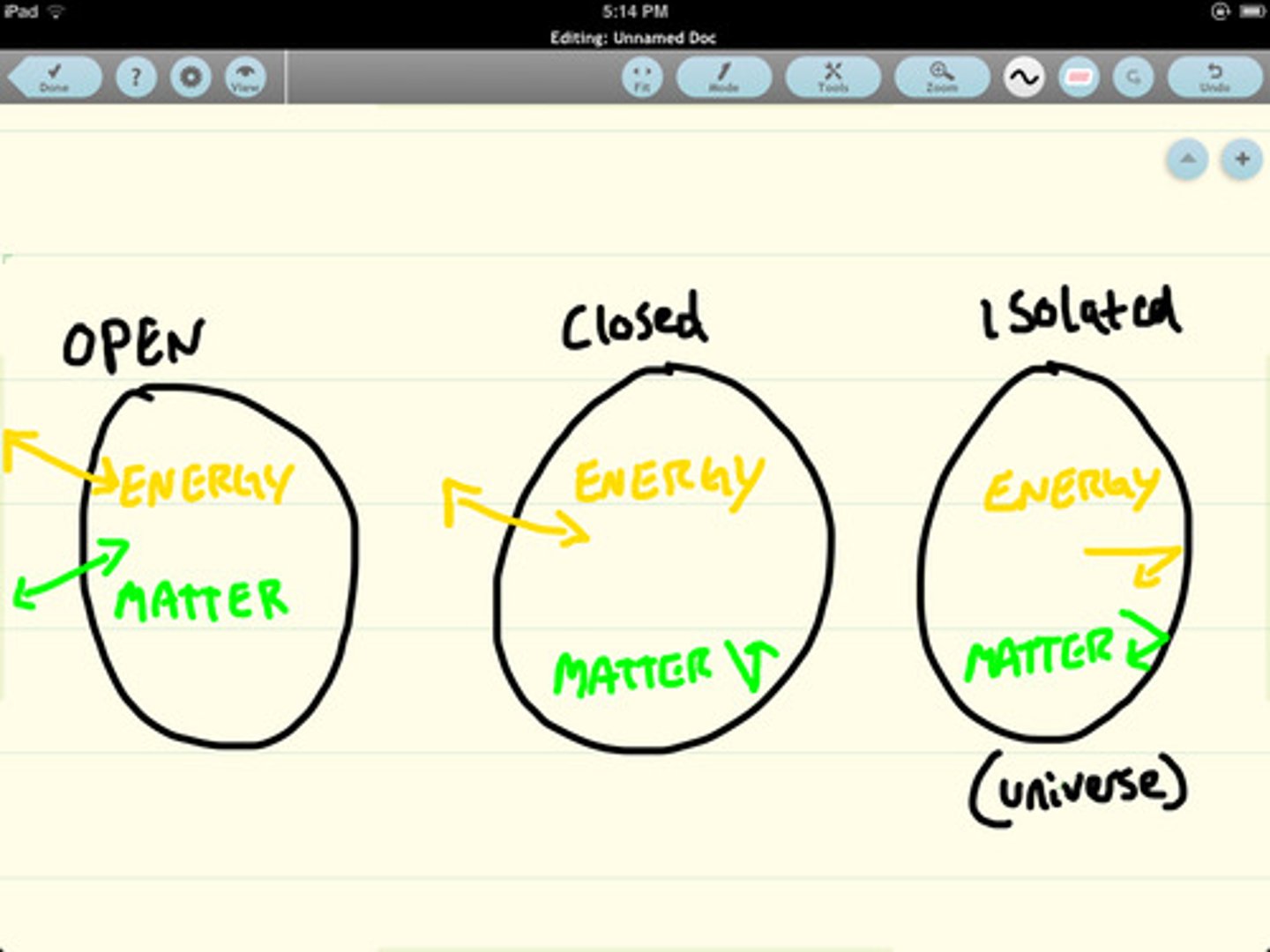
Define and give examples of open, closed, and isolated systems.
An open system exchanges both mass and energy with its surroundings, like a pot of boiling water. A closed system exchanges only energy but not matter, such as a sealed, heated bottle of water. An isolated system exchanges neither mass nor energy, with a perfectly sealed and insulated thermos being a hypothetical example
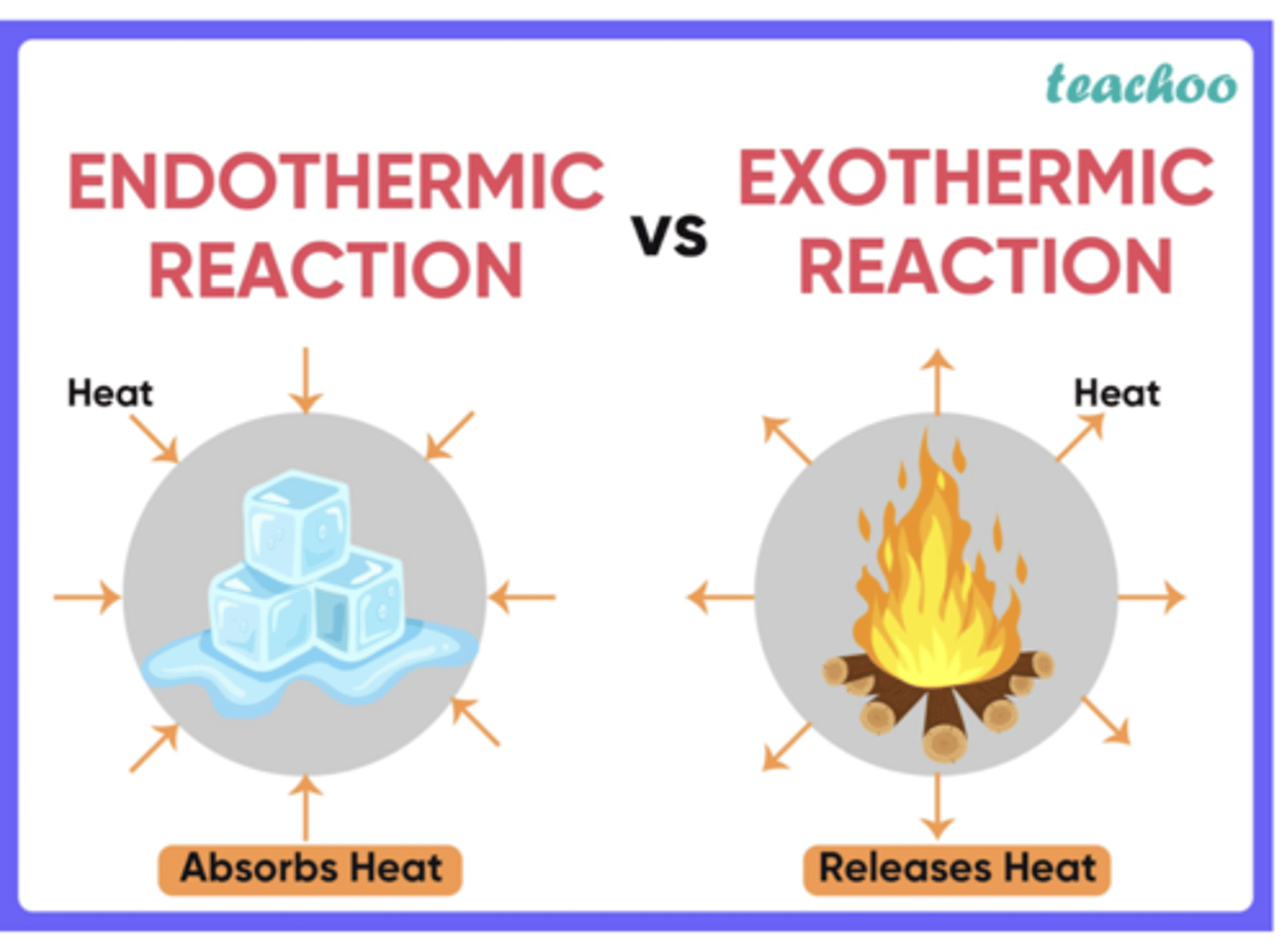
For exothermic and endothermic processes, identify the direction of thermal energy change and the sign of q or ΔH.
Exothermic processes, thermal energy flows out of the system into the surroundings, resulting in a negative sign for ΔH and q
Endothermic processes, thermal energy flows into the system from the surroundings, leading to a positive sign for q and ΔH.
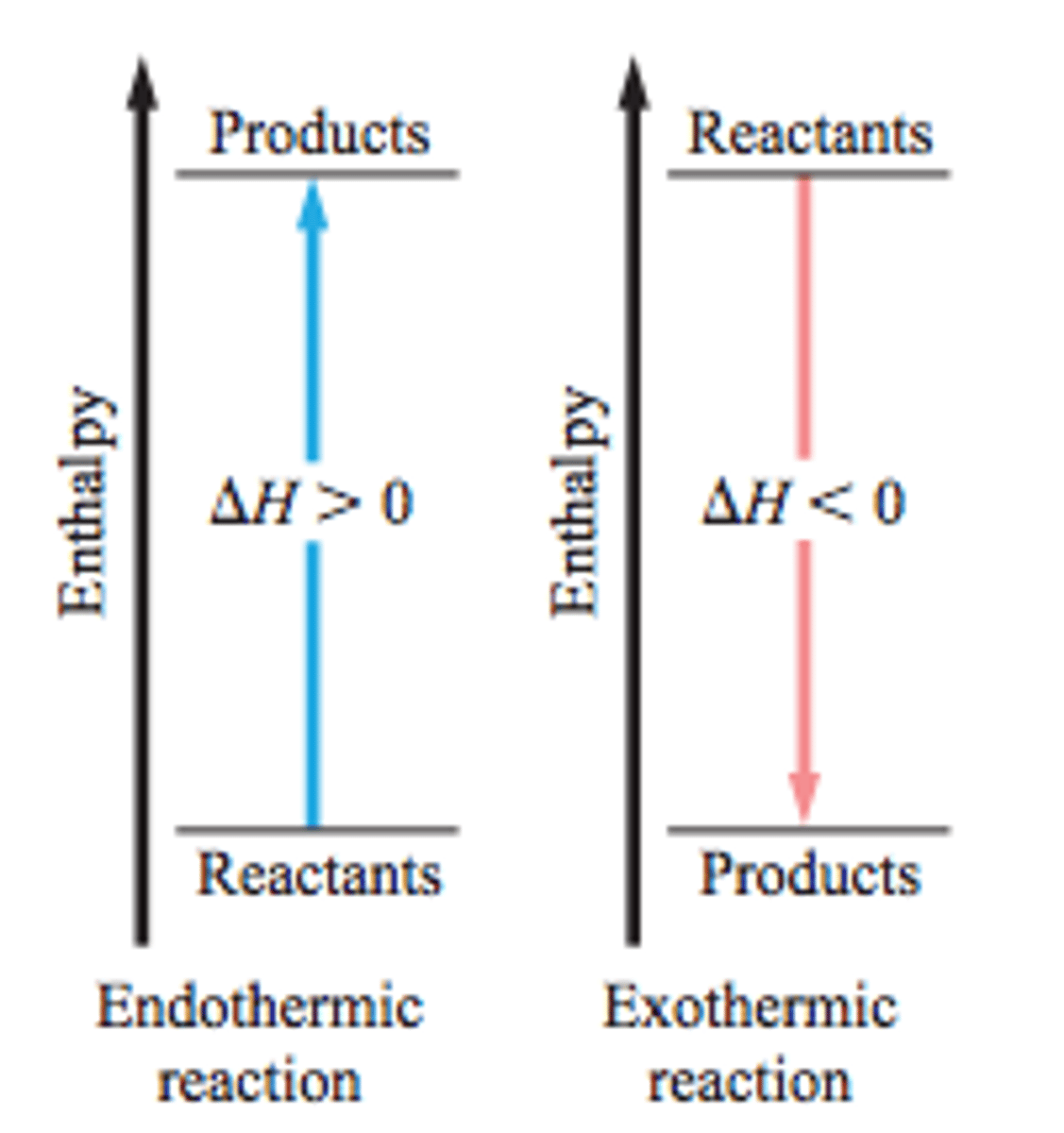
How do you interpret an enthalpy diagram?
If products are lower in energy, the reaction is exothermic (releases heat); if products are higher in energy, the reaction is endothermic (absorbs heat)
What is specific heat capacity?
The amount of energy required to increase the temperature of 1 unit of mass by 1C.
q = cmΔT
Manipulate qlost + qgained = 0 to solve for m, c, T, and/or q
m = q/ (cΔT)
c = q / (mΔT).
ΔT = q / (mc)
q = mcΔT
Distinguish between qrxn and H
qrxn: Qrxn is the measured quantity of heat exchanged in a specific, ongoing chemical reaction
ΔH: enthalpy change
how to calculate Ccal
Ccal = Q / ΔT
How to determine H from calorimeter data
calculate the heat (q) absorbed or released by the solution using q = mcΔT. Next, find the number of moles (n) of the limiting reactant and divide the heat (q) by the moles to find the molar enthalpy change: ΔH = -q / n
Still learning (1)
You've started learning these terms. Keep it up!