antigens and immunoglobulins
1/38
Earn XP
Description and Tags
week 5 clinical immunology
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
immunoglobulin (Ig) structure
heavy and light chains contain amino terminal variable (V) region that consists of 100-110 AAs (differs between ABs)
constant C regions exhibit limited variation and define the 2 light chain subtypes and the 5 heavy chain subtypes
some heavy chains (α, γ, δ) also contain proline-rich hinge region
amino terminal portions, corresponding to the V regions, bind to the antigen
effector functions are mediated by other domains
the μ and ε heavy chains (lack hinge region) contain additional domain in the middle of molecule
history of Ig structure
proteolytic enzymes (proteases) were important tool in early AB structure studies
limited digestion:
papain cleaves AB molecules into 3 fragments
pepsin cuts on the carboxyl terminal side of the disulphide bonds, producing F (ab’)2 fragment, in which 2 antigen-binding arms of the AB must remain linked
proteolytic cleavage of immunoglobulins
papain cuts the AB molecules on the amino-terminal side of the disulphide bonds that link 2 heavy chains
link 2 heavy chains, releasing the 2 arms of AB molecule as 2 identical fragments that contain antigen-binding activity
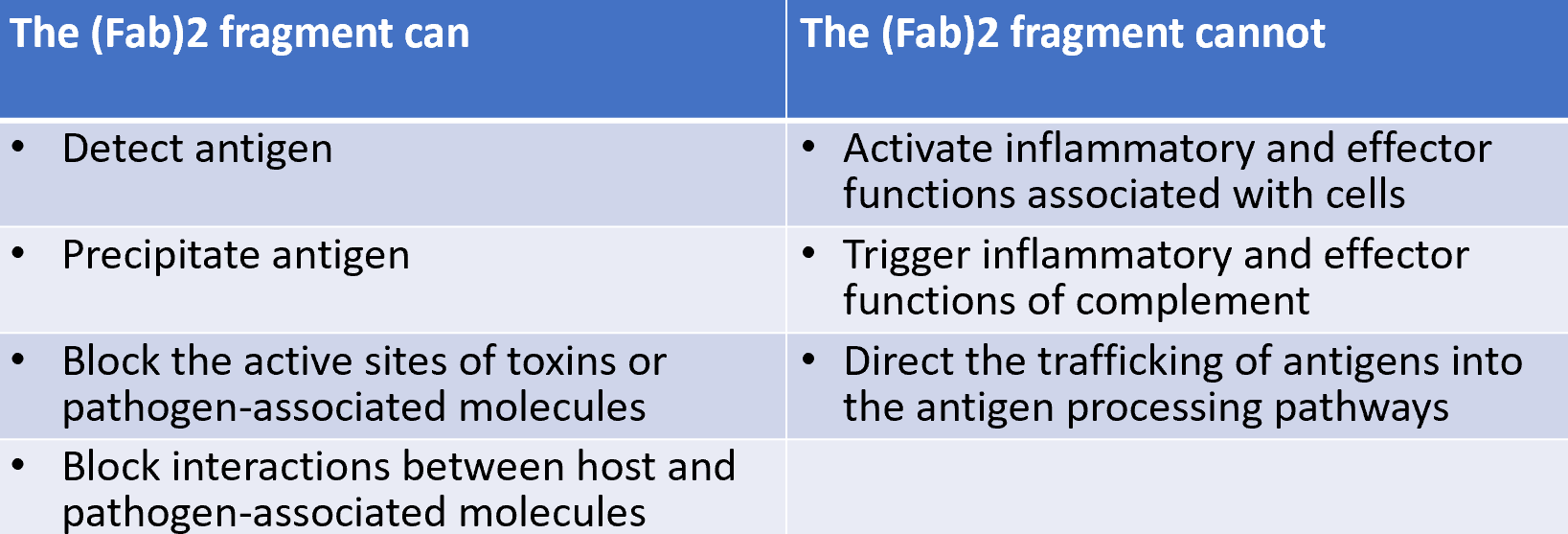
Fab fragments- fragment antigen binding
Fc fragment-fragment crystallisable
Fc fragment does not interact with antigen but rather interacts with effector molecules and cells (different between heavy chain isotypes)
pepsin cuts on the carboxyl terminal side of the disulphide bonds
pepsin cuts the remaining part of the heavy chain into several small fragments
F (ab’) 2 fragment in which the 2 antigen binding arms of the AB molecule remain linked
F (ab’)2 fragment has the same antigen-binding characteristics as AB but is unable to interact with any effector molecule
pFC’- largest remaining Fc fragment
production of monoclonal ABs
mice spleen cells immunised with antigen A produce/secrete a specific AB
spleen cells die after a few days in culture
fusing spleen cells with immortal myeloma cells by use of polyethylene glycol (PEG) results in hybrid cell line called hybridoma
HGPRT gene contributes by the spleen cell allows hybrid cells to survive in the HAT medium and only hybrid cells can grow continually in culture (selective pressure)
unfused myeloma cells and unfused spleen cells die in the HAT medium
individual hybridomas are obtained by single-cell diultion and screened for AB production
single clones that make AB of desired specificity can be isolated and grown
as each hybridoma is descended from a single cell, all cells of a hybridoma cell line make the same AB molecule hence monoclonal AB
immunoglobulin fold
characteristic structural motif of all Ig domains
sandwich of β pleated sheets
3 or 4 antiparallel strand of AA strands are connected by loops
β strands are characterised by alternating hydrophilic and hydrophobic AAs
side chains are perpendicular to the plane of paper
hydrophobic interactions stabilise the structure
disulphide bond stabilises structure
loops are where the complementarity is
hypervariable regions
also called complementarity determining regions (CDRs)
3 hypervariable regions acount for 15-209% of domain
80-85% are less variable, known as framework regions
hypervariable regions form the antigen binding site of the AB molecule
hypervariable CDRs are located on loops at the end of the Fv regions
Ig gene superfamily (IgSF)
Ig domains are not restricted to Ig genes
superfamily of related genes, particularly those encoding proteins crucial to cell-cell interactions and molecular recognition systems (MHC class I and class II molecules)
IgSF molecules are found in most cell types and are present across taxonomic boundaries
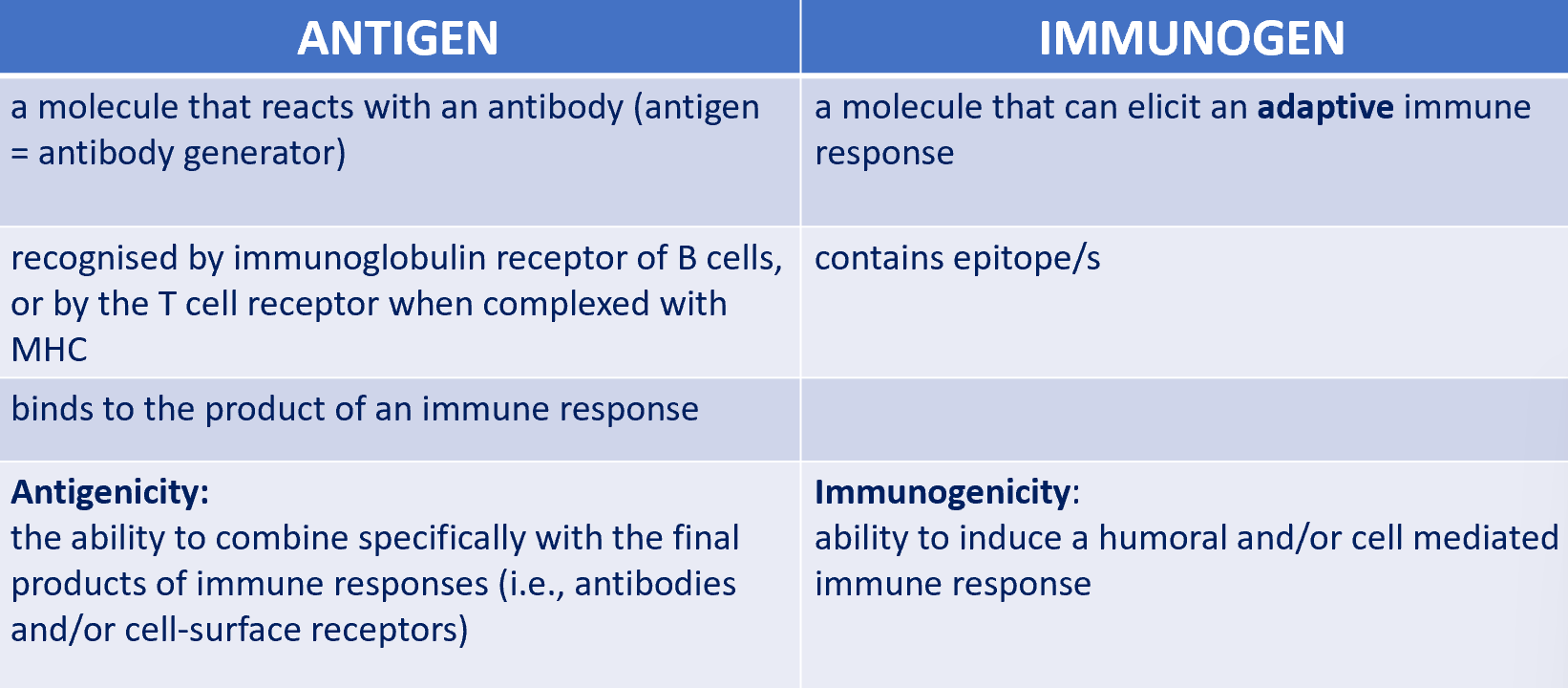
difference between antigen and immunogen
although a substance that induces a specific immune response is usually called an antigen, it’s more appropriately called an immunogen
immunogen is necessarily an antigen but an antigen may not necessarily be an immunogen
antigen vs immunogen
immunogens
induce AB production because the immune system recognises them as a threat
proteins
carbs
lipids
phospholipids
nucleic acids
only macromolecules can stimulate humoral immune response
lipid and nucleic acid must be conjugated to a carrier protein or polysaccharide to be immunogenic
antigens are not always immunogenic
immunogenicity is not always an intrinsic property
eg Bovine Serum Albumin
BSA is not immunogenic to rabbits
BSA is immunogenic to rabbits
haptens are another example
immunologists tend to use proteins or polysaccharides as immunogens in most experimental studies of humoral immunity
Hapten
a molecules that is incapable, alone, of causing the production of ABs
needs to be conjugated to a larger antigenic molecule- carrier
hapten carrier effect
immune system will then recognise the hapten as part of a larger molecule/structure
factors that affect immunogenicity
degree of dissimilarity with self molecules
molecular size
chemical composition
degradability
genotype of recipient
immune dosage
route of administration
adjuvants
adjuvant
substances that enhance the immunogenicity of an antigen in a mixture
antigen has low immmunogenicity
small amounts of antigen are available
AB response of mice to immunsisation with BSA can be increased 5 fold or more if BSA is administered with an adjuvant
the effect of adjuvants
prolonged antigen persistence
co stimulatory signals are enhanced
local inflammation increased
non specific proliferation of lymphocytes is stimulated
epitopes: the basic recognition unit
the smallest individually identifiable part of an antigen is known as an epitope
the immunologically active regions of an immunogen that interact with membrane receptors on lymphocytes or with secreted ABs
protein antigen: epitope is about 5 or 6 AA residues
carb antigen: epitope is about 5 or 6 hexose units
antigens can be classified in order of their origins
exogenous antigens
endogenous antigens
exogenous antigens
entered the body from the outside (inhalation, ingestion, injection)
taken up by endocytosis or phagocytosis
processed into fragments in the APCs
endogenous antigens
generated within the cell
normal cell metabolism
viral or intracellular bacterial infection
cancer, tumour antigens
continuous and discontinuous epitopes
an epitope on a protein antigen may involve elements of the primary, secondary tertiary and quaternary structure of the protein
continuous/ linear epitopes consist of continuous AA residues on a protein sequence
discontinuous/conformational epitopes consist of AA residues that are discontinuous in the protein sequence but are brought together in the 3D structure
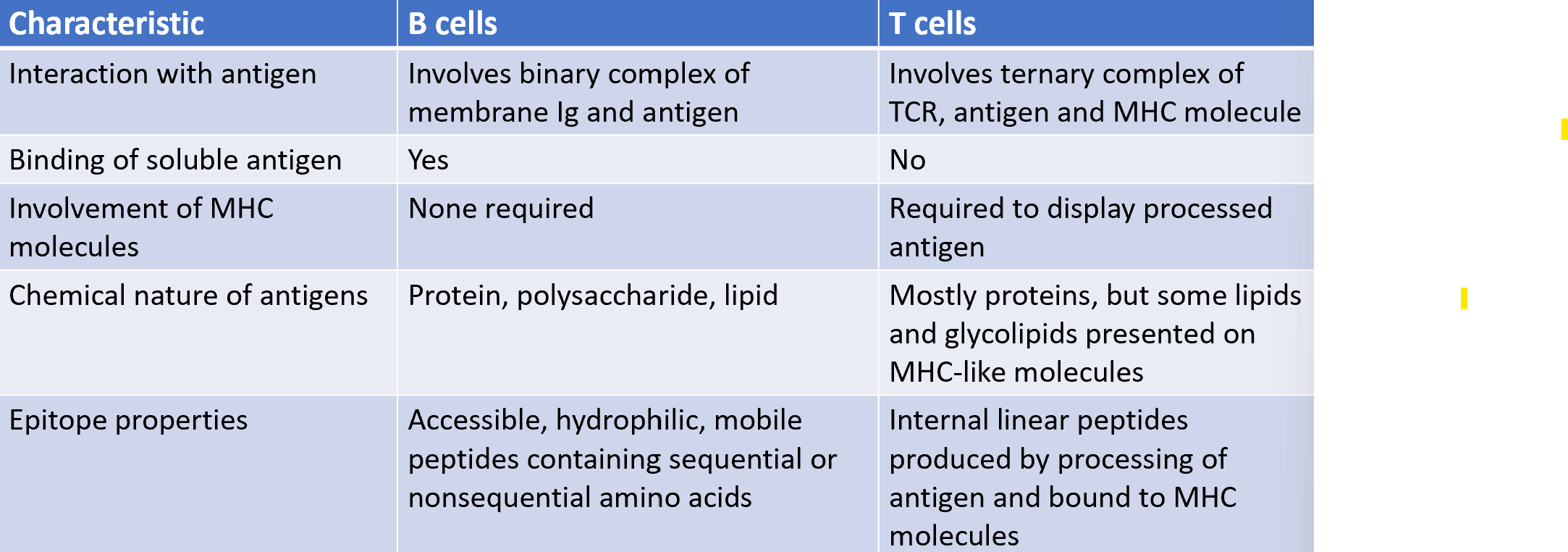
antigen recognition by T cells vs B cells
paratope
3D structure formed by the complementary binding of the tip part of variable regions (light and heavy chains) of the AB
5-10 AAs that recognise and bind the epitope of an antigen
present in the antibody’s Fv region
fragment antigen binding (Fab region)
antibody-antigen binding is the paratope-epitope interaction
4 types of noncovalent forces hold together the antigen-antibody complex
hydrogen bonds
VdWs
hydrophobic interactions (account for most of the binding energy)
electrostatic interactions occur between charged AA side chains as in salt bridges
affinity
strength of binding of one molecule to another at a single site, such as the binding of a monovalent Fab fragment of AB to a monovalent antigen
strong interaction depends on a very close fit between antigen and AB
high degree of complementarity between antigen and AB
exquisite specificity
Ag + Ab ⇌ Ag-Ab
Ka = [Ag-Ab] / [Ab] [Ag]
Kd = [Ab] [Ag] / [Ab-Ag
quantifying AB-antigen interactions
low affinity Ag-Ab complexes have Ka values between 10^4 and 10^5 L/mol
high affinity Ag-Ab complexes have Ka values as high as 10^11 L/mol
very stable complexes have very low Kd values
low affinity ABs bind antigen weakly and dissociate readily
high affinity Ab bind Ag more strongly and remain bound longer
avidity: strength of Ab-Ag attachment
the sum total of the strength of binding of 2 molecules (eg Ab and Ag) or cells to one another at multiple sites
distinct from affinity which is the strength of binding of one site on a molecule to its ligand
high avidity can compensate for low affinity
better measure within biological systems
eg IgM can interact at 10 different binding sites
AB cross-reactivity
cross reactivity occurs if 2 different antigens share an identical or very similar epitope (lower affinity than for the original epitope)
eg Streptococcus pyogenes expresses cell wall proteins called M antigens
ABs against streptococcal cell wall antigens cross react with antigens on heart tissue
some ABs cross react with the heart valves others do not
an epitope in the heart is structurally similar but not identical to bacterial epitope
superantigens bind directly to TCRs and MHC molecules
distinct class of antigens that stimulate a primary T cell response
recognised by T cells without being processed into peptides that are captured by MHC molecules
activation of superantigens causes massive production of cytokines by CD4 T cells
cytokine in turn cause systemic toxicity and suppression of the adaptive immune response
eg: staph enterotoxins, food poisoning
5 main classes of immunoglobulin isotypes
five different heavy-chain constant regions: μ, δ, γ, ε, α
each of these 5 different heavy chains is called an isotypes
heavy chain determines the class
IgM (μ)
IgG (γ)
IgA (α)
IgD (δ)
IgE (ε)
Fc structure is common to all specificities of AB within an isotype (although there are allotypes)
different classes activate distinct effector mechanisms in response to an antigen, triggering different elements of the innate immune system
immunoglobulin M (IgM)
IgM is the first AB secreted by the adaptive immune system
monomeric IgM is a heterotetramer of approx 180kDa
secreted form of IgM exists predominantly in a pentametric configuration with a molecular weight greater than 900 kDa
participates in neutralisation and clearance of pathogens
powerful activator of complement
helps activate inflammation, opsonisation and destruction of pathogens
immunoglobulin D (Igd)
IgD is co expressed with IgM on B cells due to differential RNA splicing
conjugation of IgD with antigen can activate, delete or anergise B cells
IgD plasma cells are found in the respiratory mucosa
IgD can bind to basophils and mast cells and activate these cells to produce antimicrovial factors to participate in respiratory immune defence in humans
also stimulates basophils to release B cell homeostatic factors
immunoglobulin A (IgA)
crucial role in the immune function of mucous membranes
up to 15% of total immunoglobulins produced throughout the body
IgA exists in 2 subclasses (IgA1 and IgA2)
can be produced as a monomeric or dimeric form
the IgA dimeric form is the most prevalent also called secretory IgA (slgA)
IgA1 is mostly found in serum and made by bone marrow B cells
IgA2 is mostly found in mucosal secretions, colostrum and milk and is made by B cells located in the mucosa
slgA provides a line of defence against bacteria such as salmonella, cholera, gonorrhoea and viruses like polio, flu and reovirus
secretory IgA (slgA) and transcytosis
IgA adsorbs on the layer of mucus covering the epithelium
in addition to aggregating commensal bacteria, it can neutralise pathogens and toxins preventing their access to tissues and inhibiting their functions
pathogens and toxins internalised by the epithelial cell can meet and be neutralised by IgA in endosomes
toxins/pathogens encounter pathogen specific IgA in lamina propria, resulting complexes are exported across the epithelial cell as the dimeric IgA is secreted
antigen bound to SlgA in the lumen can bind via carb residues on the Fc portion of IgA to dectin-1 on M cells in Peyer’s patches and be transported to underlying dendritic cells
immunoglobulin E (IgE)
only found in mammals
synthesised by plasma cells
utilised during immune defense against certain protozoan parasites such as plasmodium falciparum
IgE has an essential role in type I hypersensitivity- associated with allergy
Ig E also plays a pivotal role in responses to allergens
immunoglobulin G: most common type of Ig
approx 75% of serum ABs in humans
provides long term protection
important role in the elimination of microbial pathogens (agglutination, opsonisation, neutralisation, activation of the classical complement pathway, AB-dependent cellular cytotoxicity and intracellular AB mediated proteolysis- direct marked virions to the proteasome in the cytosol)
associated with type II and type III hypersensitivity reactions
4 IgG subclasses (IgG1, 2, 3 and 4) in humans that differ in their constant region which binds to IgG-Fc receptors (FcγR) and C1q
different subclasses have different effector functions
IgG1 and IgG3 ABs are generally induced in reesponse to protein antigens whereas IgG2 and IgG4 are associated with polysaccharide antigens
Fc receptors for IgG
family of Fc receptors for IgG (FcγRs) is broadly expressed by cells of haematopoietic origin
can be acitvating or inhibiting
FcγRI is an activating receptor with high affinity for IgG and is expressed on monocytic DCs and on monocytes/macrophages
FcγRIIb is an inhibitory receptor expressed on B cells, DCs and basophils
Fc receptor binding to ABs stimulates phagocytic or cytotoxic cells to destroy microbes or infected cells by ADCP or ADCC
ABs can protect against infectious agents by 5 mechanisms:
agglutination
neutralisation
opsonisation
activation of complement
AB dependent cell mediated cytotoxicity
factors affecting AB functional response
activation threshold at AB doses that vary per effector mechanism
why ABs need an Fc region
Fc region of AB interacts with cell surface receptors called Fc receptors and some proteins of the complement system
this property allows ABs to activate the immune system