3: Chemical basis of life
Units of Matter
Matter — Anything that has mass and occupies space.
Substance — A specific type of matter with a definite composition.
Element — A pure substance that cannot be broken down into simpler substances by chemical means.
Compound — A pure substance formed by the chemical combination of two or more different elements in fixed proportions. This can be broken down or decomposed into elements.
Atom — The smallest unit of matter that retains the properties of an element.
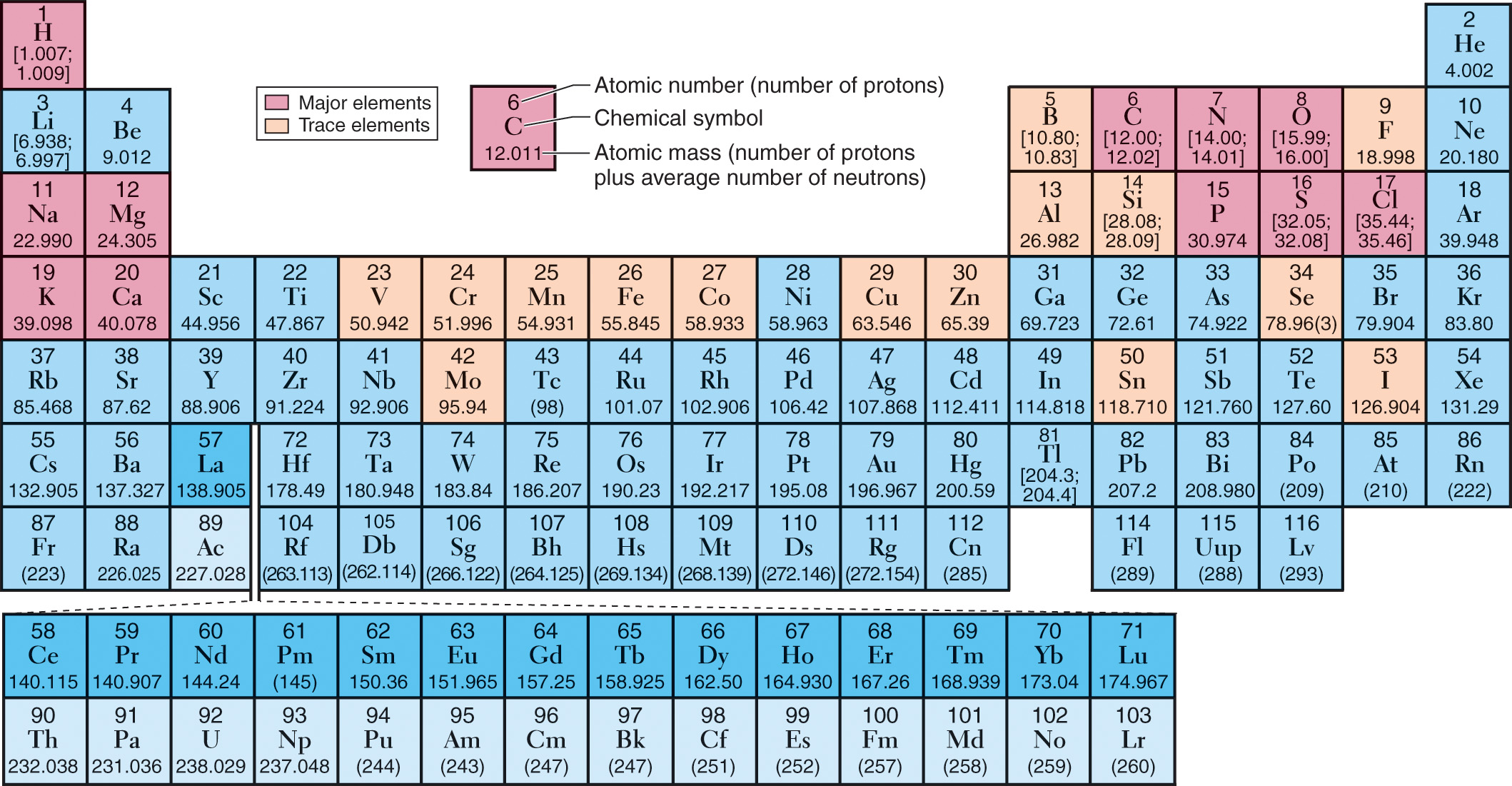
ELEMENT | HUMAN BODY WEIGHT (%) | IMPORTANCE |
Major Elements | ||
Oxygen (O) | 65.0 | Necessary for cellular respiration; component of water |
Carbon (C) | 18.5 | Backbone of organic molecules |
Hydrogen (H) | 9.5 | Component of water and most organic molecules; necessary for energy transfer and respiration |
Nitrogen (N) | 3.3 | Component of all proteins and nucleic acids |
Calcium (Ca) | 1.5 | Component of bones and teeth; triggers muscle contraction |
Phosphorus (P) | 1.0 | Principal component in the backbone of nucleic acids; important in energy transfer |
Potassium (K) | 0.4 | Principal positive ion within cells; important in nerve function |
Sulphur (S) | 0.3 | Component of many energy-transferring enzymes |
Sodium (Na) | 0.2 | Important positive ion surrounding cells |
Chlorine (Cl) | 0.2 | Important negative ion surrounding cells |
Magnesium (Mg) | 0.1 | Component of many energy-transferring enzymes |
Trace Elements | ||
Silicone (Si) | <0.1 | Uncertain |
Aluminium (Al) | <0.1 | Uncertain |
Iron (Fe) | <0.1 | Critical component of haemoglobin in the blood |
Manganese (Mn) | <0.1 | Component of many energy-transferring enzymes |
Fluorine (Fl) | <0.1 | Hardens crystals that form teeth and bones |
Vanadium (V) | <0.1 | Uncertain |
Chromium (Cr) | <0.1 | Alters insulin (hormone) effects that regulate carbohydrate lipid and protein metabolism |
Copper (Cu) | <0.1 | Key component of many enzymes |
Boron (B) | <0.1 | May strengthen cell membranes; plays a role in brain and bone development |
Cobalt (Co) | <0.1 | Component of vitamin B 12 |
Zinc (Zn) | <0.1 | Key component of some enzymes |
Selenium (Se) | <0.1 | Component of an antioxidant enzyme |
Molybdenum (Mo) | <0.1 | Key component of some enzymes |
Tin (Sn) | <0.1 | Uncertain |
Iodine (I) | <0.1 | Component of thyroid hormone |
Atoms
Atom — The smallest unit of matter that retains the properties of an element.
John Dalton — proposed the concept that matter is composed of atoms, regardless of its form.
Subatomic particle — A particle smaller than an atom that constitutes the atom.
Nucleus — The dense central core of an atom containing protons and neutrons.
Proton — A positively charged subatomic particle found in the nucleus.
Neutron — A neutral subatomic particle found in the nucleus.
Electron — A negatively charged subatomic particle that orbits the nucleus.
Atomic Structure
Cloud Model
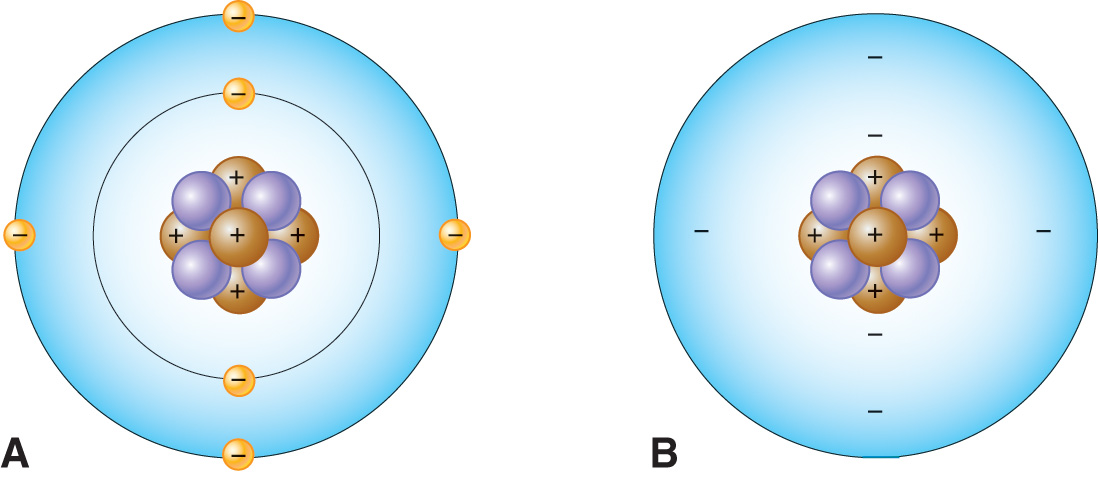
Atoms contain different kinds of smaller or subatomic particles that are found in either a central nucleus or its surrounding “electron cloud” or “field”.
Model of an Atom
Nucleus — the core of an atom
Electron Shells — energy levels or outer region of an atom where electrons inhabit.
Electron Clouds — the area around an atom's nucleus where electrons are most likely to be found.
The number of negatively charged electrons moving around an atom’s nucleus equals the number of positively charged protons in the nucleus.
The opposite charges therefore cancel or neutralize each other, which means atoms are electrically neutral particles.
Atomic number and mass number
Atomic number — The number of protons in the nucleus of an atom.
Mass number — The sum of the number of protons and neutrons in the nucleus of an atom.
Atomic mass — Another term for mass number.
Because protons and neutrons weigh almost exactly the same, the equation for determining mass number is as follows:
Mass Number = ( p+ + n0 )
Energy Levels
Electron cloud model — Emphasizes the probabilistic nature of electron location.
Probability distribution — The mathematical function that describes the likelihood of finding an electron at a particular point in space.
Bohr model — Provides a simplified, visual representation of electron arrangement in energy levels.
Properties
Exhibits electrons in concentric circles showing relative distances of the electrons from the nucleus
Each ring or shell represents a specific energy level and can hold only a certain number of electrons
Number and arrangement of electrons determine whether an atom is chemically stable
Outermost energy level — The highest energy level of an atom that contains electrons.
Stable electron configuration — An atom with a filled outermost energy level (usually eight electrons).
Electron shell — A region of space surrounding the nucleus where electrons are likely to be found.
Energy level — A specific region of an atom where electrons have a particular amount of energy.
Niels Bohr — A Danish physicist who proposed a model of the atom where electrons orbit the nucleus in specific energy levels. He won the Nobel Prize in Physics in 1922 for his contributions to the understanding of atomic structure.

Chemical bond — A force that holds atoms together in a molecule or compound.
Octet rule — The tendency of atoms to gain, lose, or share electrons to achieve a stable electron configuration of eight electrons in the outermost energy level.
Inert element — An element that is chemically unreactive due to a stable electron configuration.

Isotopes
Isotope — Atoms of the same element with the same number of protons but different numbers of neutrons.
Atomic weight — The average mass number of an element based on the abundance of its isotopes in nature.
The atomic nuclei of more than 99% of all carbon atoms in nature have six protons and six neutrons.
Carbon-13 — an isotope of carbon that has seven neutrons instead of six.
It makes up about 1% of the world’s carbon atoms.
Carbon-14 — an isotope of carbon that has eight neutrons instead of six.
It is unstable and undergoes nuclear breakdown.
Radioactive isotope (radioisotope) — An unstable isotope that undergoes nuclear decay, emitting particles and radiation.
Decay — The process by which a radioactive isotope breaks down, releasing particles and radiation.
Radioactivity — The emission of radiation from an atom’s nucleus. It differs from chemical activity because it can change the number of protons in an atom.
Alpha Particles — Are heavy particles consisting of two protons plus two neutrons.
Beta Particles — Are electrons formed in a radioactive atom’s nucleus by one of its neutrons breaking down into a proton and an electron.
These travels musch greater speed than alpha particles.
Gamma Rays — Are electromagnetic radiation, a form of light energy.
Chemical Bonds
Chemical Reaction — A process that involves the rearrangement of atoms to form new substances with different properties.
Chemical Bonds — A process formed from reactions that hold atoms together.
Crystal — A solid material composed of atoms arranged in a regular, repeating pattern.
Carbon dioxide — a molecule composed of one carbon atom and two oxygen atoms.
Water — a molecule composed of two hydrogen atoms and one oxygen atom.
Ionic Bonds
Ionic Bond — A chemical bond formed by the transfer of electrons between atoms. Also called electrovalent bond.
It occurs as a result of the attraction between atoms that have become electrically charged by the loss or gain of electrons.
Ion — An atom that has gained or lost electrons, resulting in a net electrical charge. Can also be called, electrolytes.
Cation — A positively charged ion, formed by losing electrons.
Anion — A negatively charged ion, formed by gaining electrons.
Example of Ionic Bond:
Sodium chloride (NaCl) — a compound formed by the ionic bonding of sodium cations (Na⁺) and chloride anions (Cl⁻).
Electron Transfer — The process by which one atom donates an electron to another atom.
Electrostatic Force — The attractive force between oppositely charged ions.
Covalent Bonds
Covalent Bond — A chemical bond formed by the sharing of electrons between atoms.
Single Covalent Bond — A covalent bond where one pair of electrons is shared.
Double Bond — A covalent bond where two pairs of electrons are shared.
Examples of Covalent Bond:
Hydrogen gas (H₂) — a molecule formed by a single covalent bond between two hydrogen atoms.
Carbon dioxide (CO₂) — a molecule formed by double covalent bonds between a carbon atom and two oxygen atoms.
Hydrogen Bonds
Hydrogen Bond — A weak attraction between a hydrogen atom bonded to a highly electronegative atom (oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule or part of the same molecule.
Polar Molecule — A molecule with regions of positive and negative partial charges due to unequal electron distribution.
Water — A polar molecule capable of forming hydrogen bonds with other water molecules.
Nonpolar Molecule — A molecule with electrons shared equally among atoms, resulting in no polarity.
Oil — A nonpolar molecule that does not form hydrogen bonds with water.
Polar molecules are attracted to each other through hydrogen bonds, while nonpolar molecules tend to interact with other nonpolar molecules. This explains the separation of oil and water.
Chemical Reactions
Chemical Reaction — A process involving the rearrangement of atoms to form new substances.
Reactants — The starting substances in a chemical reaction.
Products — The substances formed as a result of a chemical reaction.
Synthesis Reaction — A reaction where two or more reactants combine to form a more complex product. This requires energy input.
Formula : A + B → AB
Example: Amino acids combining to form proteins.
Decomposition Reaction — A reaction where a complex substance breaks down into simpler substances. This reaction releases energy.
Formula : AB → A + B + Energy
Example: Breakdown of nutrients to release energy.
Exchange Reaction — A reaction where two reactants exchange components to form two new products.
Formula : AB + CD → AD + CB
Example: Reaction between lactic acid and sodium bicarbonate.
Reversible Reaction — A reaction that can proceed in both directions.
Formula : A + B ⇌ AB
Example: The process of photosynthesis in plants
Metabolism
Metabolism — The sum of all chemical reactions occurring in the body.
Also called body chemisty.
Catabolism — Chemical reactions that break down complex molecules into simpler ones, releasing energy.
Hydrolysis — A catabolic reaction that uses water to break down molecules.
Examples:
Hydrolysis of Fat Molecule → Glycerol and Fatty Acid Molecules.
Sucrose → Glucose and Fructose
Protein Hydrolysis → Amino Acids.
Adenosine triphosphate — a molecule that stores and transfers energy.
Anabolism — Chemical reactions that build complex molecules from simpler subunits.
Dehydration Synthesis — An anabolic reaction that removes water to join molecules together.
Examples:
Carbohydrates
Lipids
Proteins
Nucleic Acids.
Organic and inorganic compounds
Biomolecules — Large organic molecules essential for life.
Organic Compound — A compound containing carbon-carbon (C-C) or carbon-hydrogen (C-H) covalent bonds.
Inorganic Compound — A compound typically not containing carbon or, if it does, lacking C-C or C-H bonds.
Organic molecules are generally larger and more complex than inorganic molecules.
Both organic and inorganic compounds are vital for the chemistry of life.
Inorganic molecules
Water
Water is essential for all living organisms.
It makes up a significant portion of the human body.
The chemistry of life is largely based on the properties of water.
Properties of Water
Solvent — A substance capable of dissolving other substances.
Solute — A substance dissolved in a solvent.
Polarity — The property of having a positive and negative end.
Hydration Shell — A layer of water molecules surrounding a charged solute.
Specific Heat — The amount of heat required to raise the temperature of a substance by one degree.
PROPERTY | DESCRIPTION | EXAMPLE OF BENEFIT TO BODY |
Strong polarity | Polar water molecules attract other polar compounds, which causes them to dissociate | Many kinds of molecules can dissolve in cells, thereby permitting a variety of chemical reactions and allowing many substances to be transported |
High specific heat | Hydrogen bonds absorb heat when they break and release heat when they form, thereby minimizing temperature changes | Body temperature stays relatively constant; body chemistry facilitated |
High heat of vaporization | Many hydrogen bonds must be broken for water to evaporate | Evaporation of water in perspiration cools the body |
Cohesion | Hydrogen bonds hold molecules of water together | Water works as lubricant or cushion to protect against damage from friction or trauma |
Oxygen and carbon dioxide
Oxygen (O₂) — A diatomic molecule essential for cellular respiration.
Two oxygen atoms joined by a double covalent bond.
Required for the complete breakdown of nutrients and energy release
Carbon Dioxide (CO₂) — A simple inorganic compound involved in cellular respiration and acid-base balance.
Produced as a waste product of nutrient breakdown and plays a role in acid-base balance.
Electrolytes
Large group of inorganic compounds that includes acids, bases, and salts.
Electrolyte — A substance that dissociates into ions in solution. Can also be called ions.
Dissociation — The process of breaking down into ions.
Acids and bases
Characteristic | Acids | Bases |
Definition | Substances that release hydrogen ions (H⁺) in solution; also called proton donors. | Substances that release hydroxide ions (OH⁻) or accept hydrogen ions (H⁺) in solution; also called proton acceptors. |
Taste | Sour | Bitter |
Litmus Test | Turns red | Turns blue |
Ion Released in Solution | Releases hydrogen ions (H⁺), increasing the concentration of H⁺ in the solution. | Releases hydroxide ions (OH⁻) or reduces H⁺ concentration in the solution. |
Effect on H⁺ / OH⁻ Balance | Shifts the balance in favour of excess H⁺ ions, increasing acidity. | Shifts the balance in favour of excess OH⁻ ions, reducing acidity (increasing alkalinity). |
Strength Classification | - Strong acids dissociate completely, releasing many H⁺ ions. - Weak acids dissociate minimally, releasing few H⁺ ions. | - Strong bases dissociate completely, releasing many OH⁻ ions. - Weak bases dissociate minimally, releasing few OH⁻ ions. |
Role in the Body | - Hydrochloric acid (HCl) aids digestion in the stomach. - Acids play roles in various physiological processes through proton donation. | - Bicarbonate ion (HCO₃⁻) is crucial for respiratory gas transport and pH balance. - Bases help eliminate waste and maintain normal pH. |
Example of a Common Compound | Hydrochloric acid (HCl) | Sodium hydroxide (NaOH) |
The pH Scale
pH — A measure of the hydrogen ion concentration in a solution.
A pH of 7 indicates neutrality (equal amounts of H + and OH − )
A pH of less than 7 indicates acidity (more H + than OH − )
A pH greater than 7 indicates alkalinity (more OH − than H + )

Buffers
pH Homeostasis — The maintenance of a stable pH in body fluids.
Body fluids have a very narrow and tightly regulated pH range.
Carbonic Acid — Contributes to the slightly lower pH of venous blood.
Buffer — A substance that resists changes in pH by accepting or donating hydrogen ions.
They act as a reservoir for hydrogen ions, accepting or donating them as needed.
Salts
Salt — A compound formed by the reaction of an acid and a base.
Neutralization Reaction — A reaction between an acid and a base that produces a salt and water.
Formula: AB + CD → CB + AD
Sodium Chloride (NaCl) — A common salt formed from hydrochloric acid (HCl) and sodium hydroxide (NaOH).
Potassium Chloride (KCl) — A common salt formed from hydrochloric acid (HCl) and potassium hydroxide (KOH).
Chemicals Out of Balance
Hypercapnia — A condition where carbon dioxide concentration in the blood is abnormally high.
Acidosis — A condition where the body's pH is lower than normal.
High CO₂ levels can lead to acidosis, which can disrupt protein structure and function.
Toxins — Substances that can damage body molecules.
Carbon Monoxide (CO) — A toxin that binds to hemoglobin, preventing oxygen transport.
Mercury (Hg) — A toxin that damages cells by binding to sulfur-containing molecules.
Chemical Imbalance — An abnormal concentration of a particular chemical in the body.