Electron Configuration and Energy Level Diagrams (copy)
0.0(0)
Card Sorting
1/6
Last updated 5:38 PM on 11/21/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
1
New cards
Pauli exclusion principle
no two electrons can have the same set of quantum numbers
each orbital can only hold 2 electrons with opposite spins
each orbital can only hold 2 electrons with opposite spins
2
New cards
Aufbau principle
electrons will first occupy the orbital of the lowest energy level
3
New cards
Hund's rule
every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied
all electrons in singly occupied orbitals have same spin
all electrons in singly occupied orbitals have same spin
4
New cards
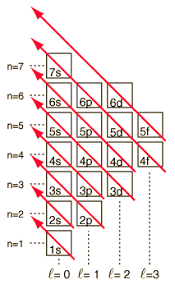
Why do we add 4s before 3d?
energy of the 4s orbital is lower than 3d
aufbau's principle states we must add electrons to lowest level
aufbau's principle states we must add electrons to lowest level
5
New cards
when dealing with ions
electrons are added or removed from orbital with greatest primary quantum number (n value)
6
New cards
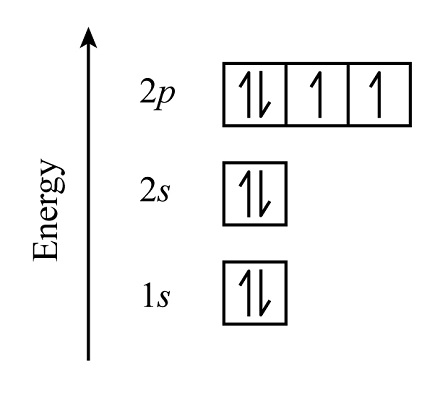
what are energy level diagrams (orbital box diagrams)
illustrations that represent electron configuration of atom/ion
must apply aufbau's principle, hund's rule, and pauli's exclusion principle
boxes represent 1 orbital and can only hold 2 electrons
drawn with up and down arrows to represent spin
must apply aufbau's principle, hund's rule, and pauli's exclusion principle
boxes represent 1 orbital and can only hold 2 electrons
drawn with up and down arrows to represent spin
7
New cards
what are degenerate orbitals
same height, fill them one at a time