ELIMINATION REACTIONS
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
74 Terms
Q: Primary RX + strong nucleophile → SN1 or SN2?
A: SN2
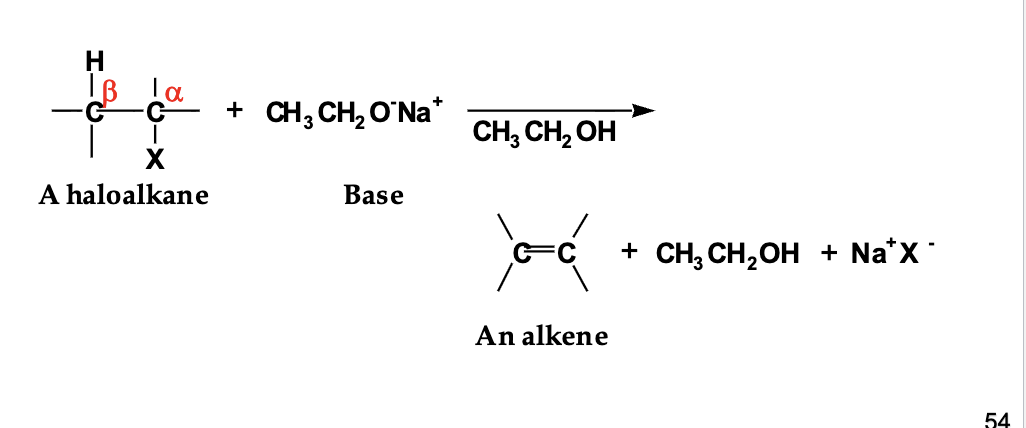
What is β-elimination (say it without looking)?
A reaction where two groups are removed from adjacent carbons (α and β), and a new C=C (π bond) forms.
In a haloalkane, how do I find the α-carbon?
The α-carbon is the carbon directly bonded to the leaving group (X).
What is a β-hydrogen?
A hydrogen attached to a carbon next to the α-carbon (one bond away from X).
Before elimination can happen, what three things must be present?
A leaving group (X)
A β-hydrogen
A base (like CH₃CH₂O⁻)
What does the base actually do in β-elimination?
The base grabs the β-hydrogen.
When the base takes the β-hydrogen, what moves where?
The C–H bond electrons move down
They form a C=C double bond between α and β carbons
The leaving group leaves
draw the arrows
🔴 Arrow 1 — Base → β-hydrogen
Arrow starts at the oxygen of CH₃CH₂O⁻
Arrow points to the β-hydrogen
👉 Meaning: the base grabs the H
🔴 Arrow 2 — C–H bond → between the carbons
Arrow starts at the C–H bond (the β-hydrogen bond)
Arrow points between the α and β carbons
👉 Meaning: those electrons form the C=C double bond
🔴 Arrow 3 — C–X bond → X
Arrow starts at the C–X bond
Arrow starts at the C–X bond
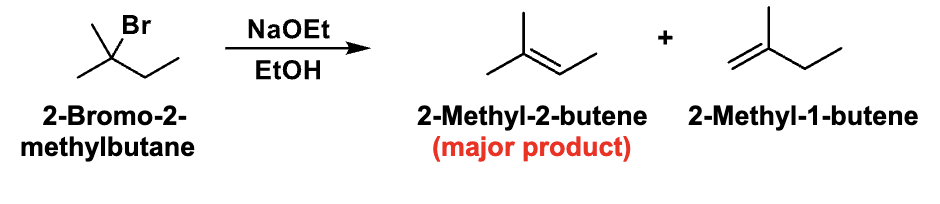
Looking at the slide, what kind of reaction is happening?
β-elimination (forming an alkene)
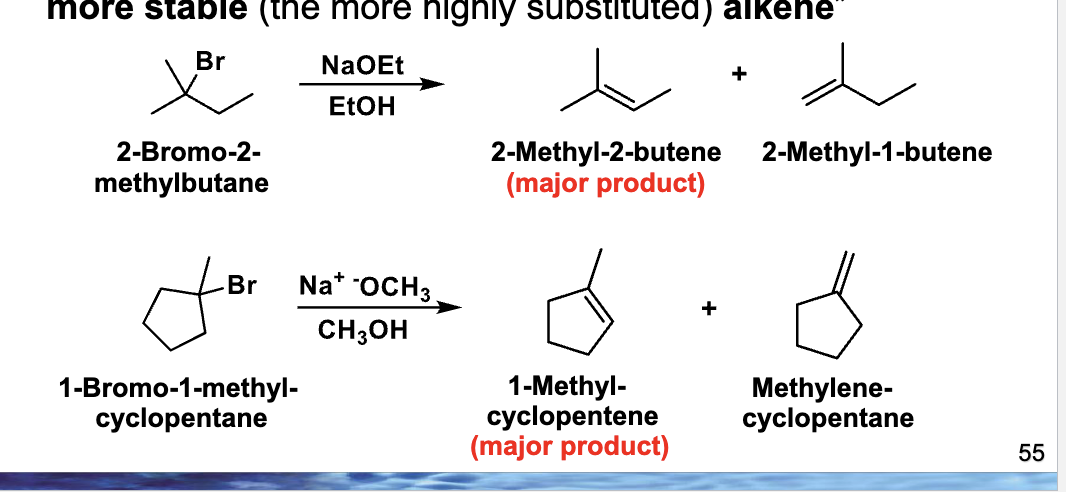
What species is doing the attacking / grabbing?
A base (like NaOEt or NaOCH₃)
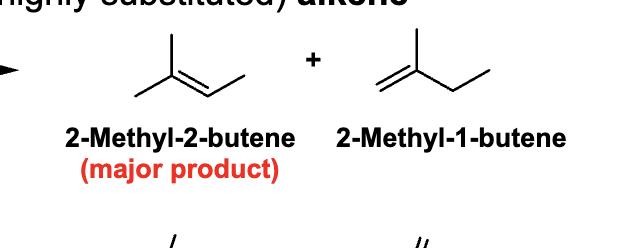
If multiple alkenes can form, which one is major?
The more substituted (more stable) alkene → Zaitsev’s rule
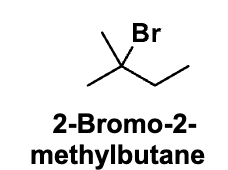
Draw 2-bromo-2-methylbutane exactly as in the slide.
Step 2: Circle things (no arrows yet)
Step 3: Choose the β-H that gives the MAJOR product
Step 4: NOW draw arrows (this is the core skill)
Circle Br
Label that carbon α
Circle the two β-carbons next to it
Circle ONE hydrogen on each β-carbon
✔ Choose the β-H that gives 2-methyl-2-butene
From oxygen of the base → β-hydrogen
✏ Meaning: base grabs H
From C–H bond → between α and β carbons
✏ Meaning: electrons form the C=C bond
From C–Br bond → Br
✏ Meaning: Br leaves
Step 5: Redraw ONLY the product

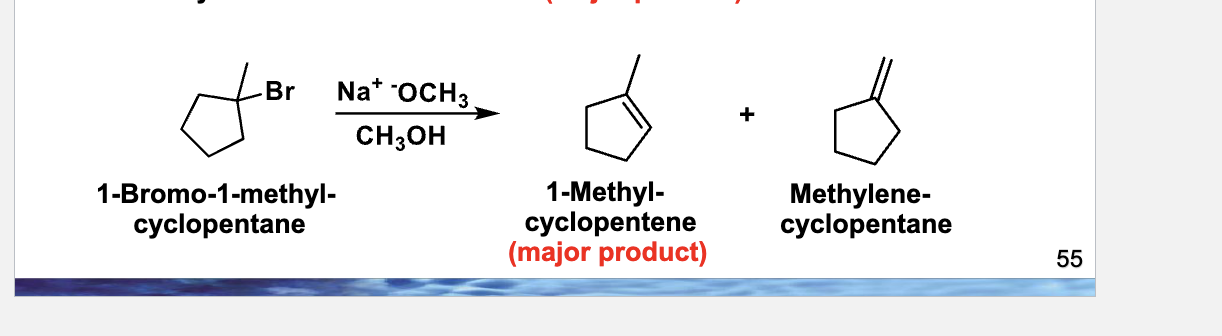
Step 1: Draw ONLY the starting molecule
Step 2: Circle + label (still no arrows)
Step 3: Predict BOTH possible alkenes (no arrows yet)
Step 4: NOW draw the arrows (THIS is the skill)
🔴 Arrow 1 — Base → β-hydrogen
Arrow starts at O⁻ of the base
Arrow points to the β-H that gives the inside-ring alkene
(Base grabs H)
🔴 Arrow 2 — C–H bond → between carbons
Arrow starts at the C–H bond
Arrow points between α and β carbons
(Electrons form the C=C)
🔴 Arrow 3 — C–Br bond → Br
Arrow starts at C–Br
Arrow points to Br
(Leaving group leaves)
Step 5: Redraw ONLY the products
You should end with:
1-methylcyclopentene ✅ (major)
Alcohol (MeOH)
Br⁻
If the double bond is inside the ring, you did it right.
What happens FIRST in E1?
The leaving group leaves → carbocation forms
What is special about E2?
Everything happens at the same time (one step, concerted)
Which mechanism NEEDS a strong base?
E2
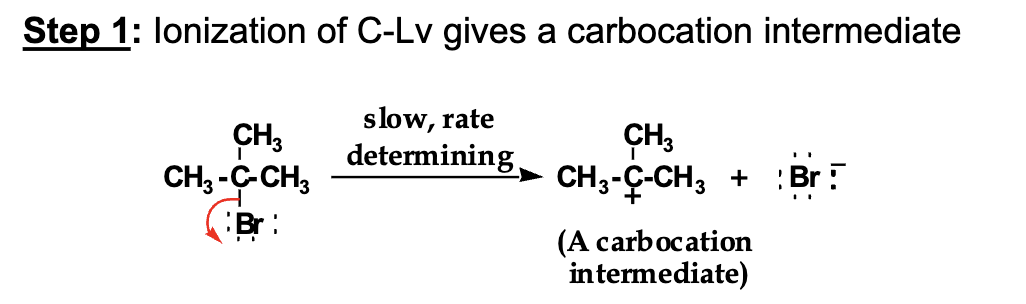
STEP 1 — LEAVING GROUP LEAVES (ONLY ELECTRONS MOVE)
What bond are we breaking?
👉 The C–Br bond
What electrons move?
👉 The two electrons in the C–Br bond
Arrow STARTS at: the C–Br bond line
Arrow ENDS at: Br
What do you draw AFTER this arrow?
Br⁻ (with lone pairs)
The carbon that lost Br now has:
only 3 bonds
no octet
a positive charge
STEP 2 — BASE REMOVES β-HYDROGEN (NOW NEW ELECTRONS MOVE)
Arrow A — base → hydrogen
What electrons move?
👉 A lone pair from the base
EXACT ARROW
Arrow STARTS at: a lone pair on the base (O:)
Arrow ENDS at: the β-hydrogen
✏ Meaning: base grabs H⁺
Arrow B — C–H bond → C=C
What electrons move?
👉 The two electrons in the C–H bond
EXACT ARROW
Arrow STARTS at: the C–H bond
Arrow ENDS at: the space between α and β carbons
✏ Meaning: those electrons form the π bond
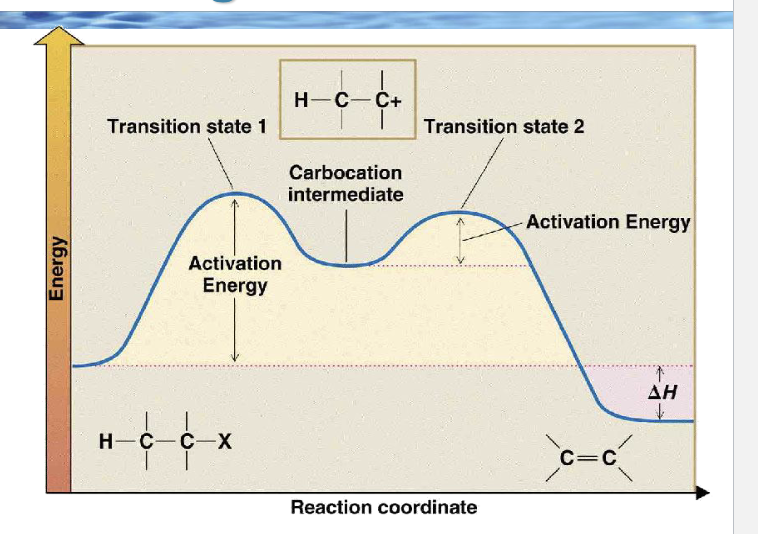
Because the first step is slow, which species controls the rate?
Only R–Lv (the substrate)
draw the reaction coordinate diagram for e1
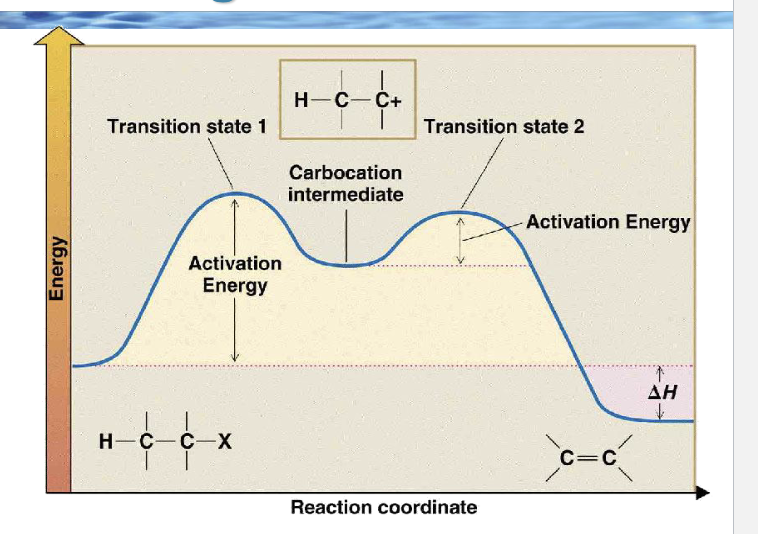

Why is the second peak lower than the first?
Because forming the alkene from a carbocation is easier than forming the carbocation
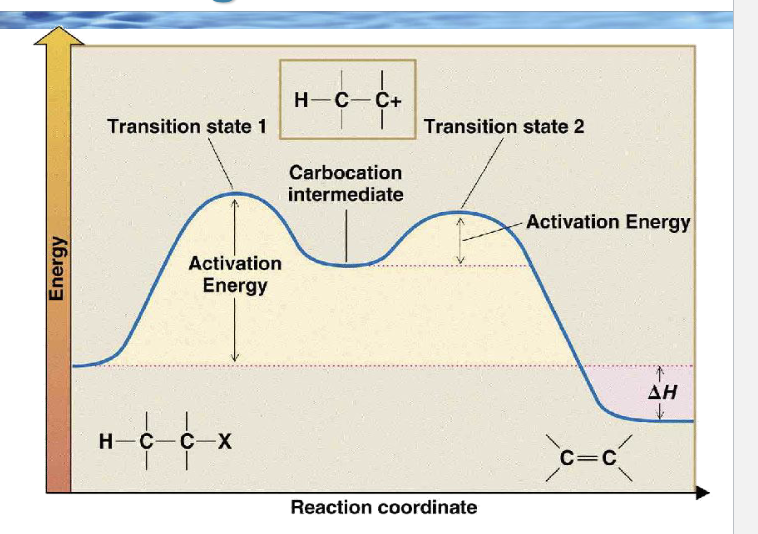
Which arrow-pushing step matches the first peak?
Which arrows match the second peak?
Arrow from C–X bond → X
Base lone pair → β-H
C–H bond → C=C
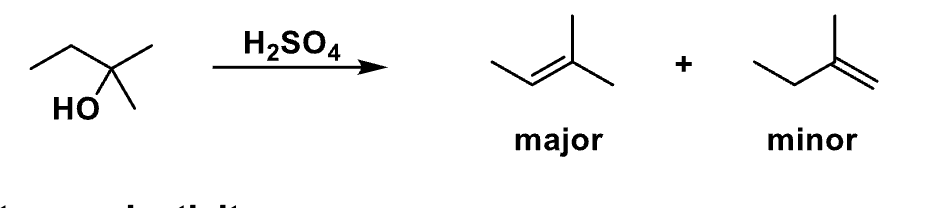
This slide is comparing different alkene positions. What type of selectivity is this?
Regioselectivity
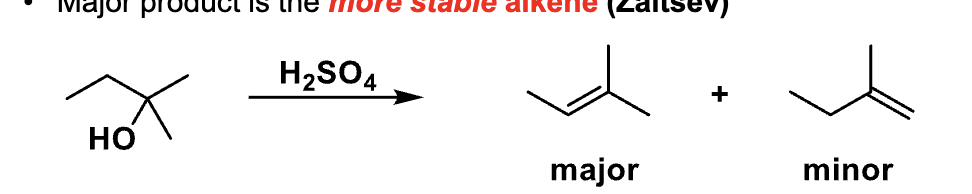
According to the slide, which alkene is favored in E1?
The more stable (more substituted) alkene → Zaitsev
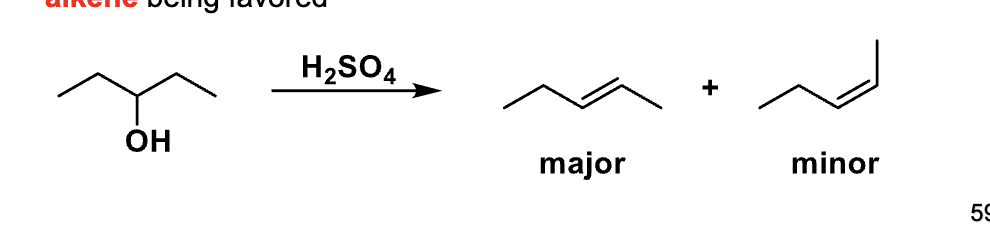
Now the slide shows cis vs trans. What kind of selectivity is this?
Stereoselectivity
Why can E1 give both cis and trans alkenes?
Because the carbocation is planar
What does a planar carbocation allow the base to do? (e1)
Remove a β-hydrogen from either side
Which alkene stereoisomer is usually the major product?
trans
What is the rate law for E2?

Which species must be involved in the rate-determining step?
Both the substrate (R–Lv) and the base

👉 Which drawing has more groups attached to the partial double bond?
A

Which transition state is lower in energy?
The one leading to the more substituted alkene
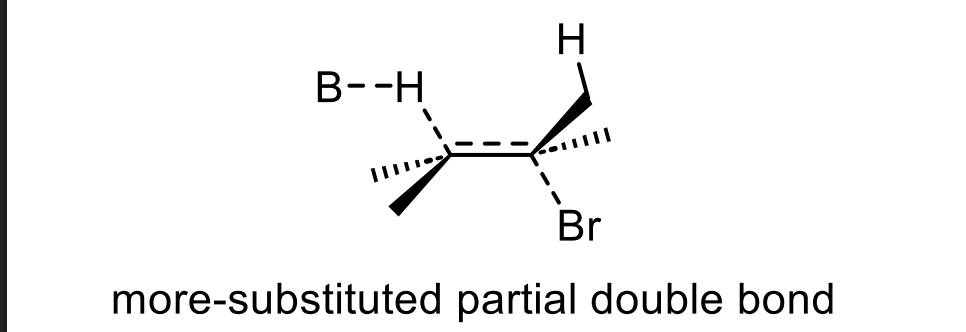
In the TOP diagram, the LEFT pathway forms the major product.
👉 Which β-hydrogen is removed — the one on the LEFT β-carbon or RIGHT β-carbon?
The LEFT β-hydrogen
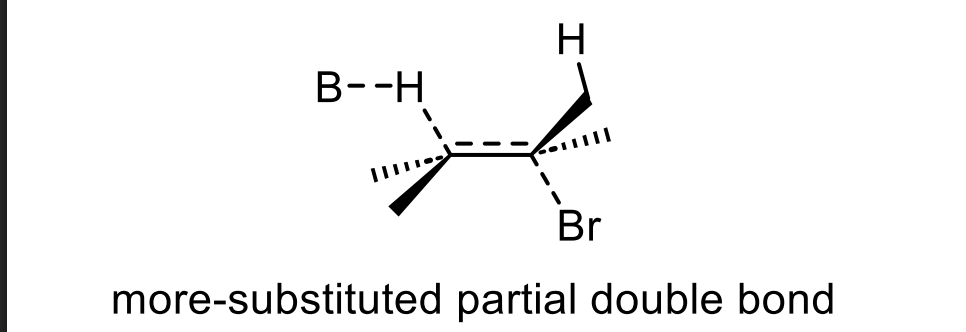
If the LEFT β-hydrogen is removed, where does the double bond form?
Between the α-carbon and the LEFT β-carbon
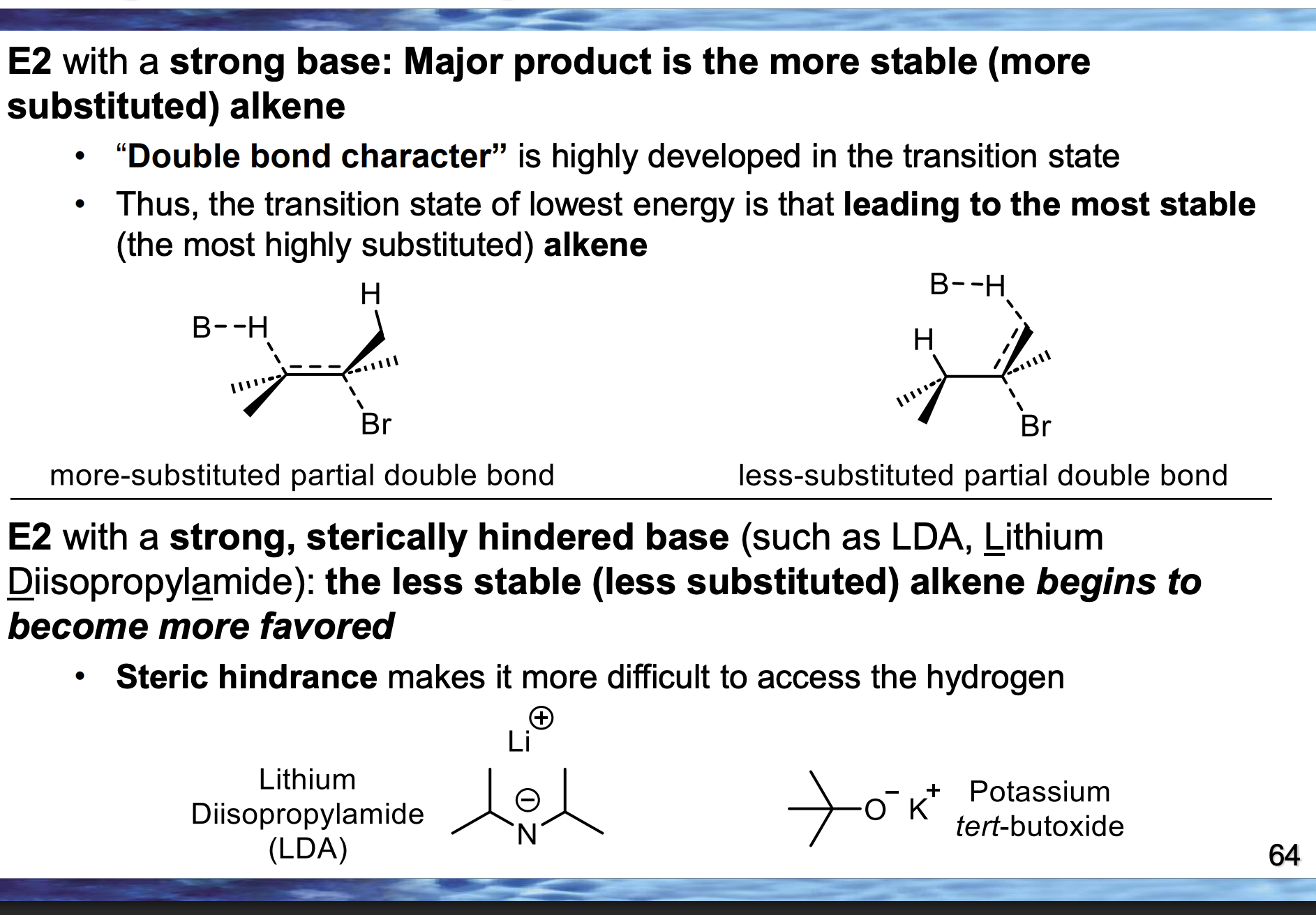
Compare the base in the bottom diagram to the top one.👉 Is the bottom base larger or smaller?
Because the bottom base has large alkyl groups around the basic atom, making it physically bigger in space.
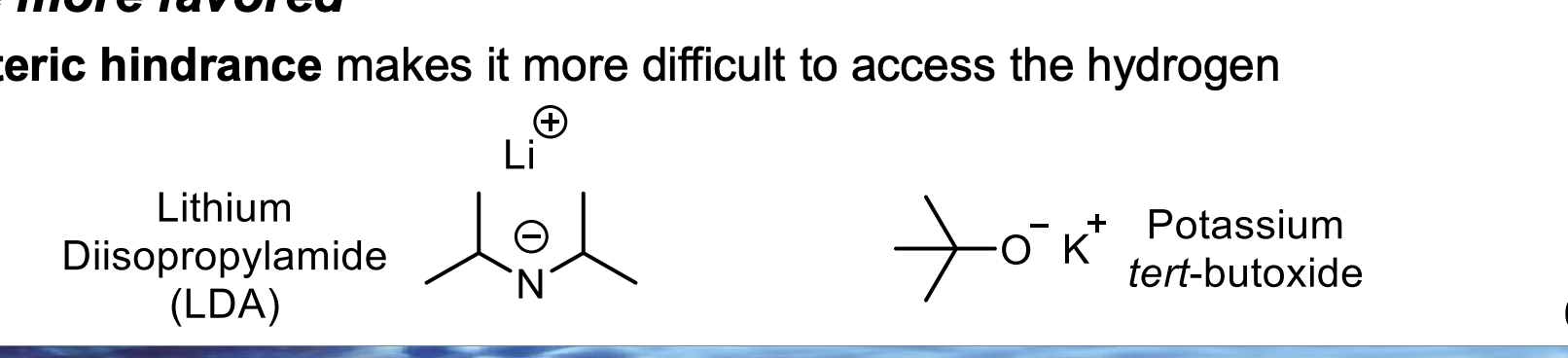
Look at the β-carbons in the BOTTOM diagram.
👉 Which side (LEFT or RIGHT) looks more crowded with carbon groups?
LEFT is more crowded.
Because the LEFT β-carbon is attached to more alkyl groups, which take up space and block access.
Given that the base is bulky, which β-hydrogen is easier to physically reach — LEFT or RIGHT?
RIGHT.
Because the RIGHT β-hydrogen is on a less substituted (less crowded) carbon, so there is more open space.

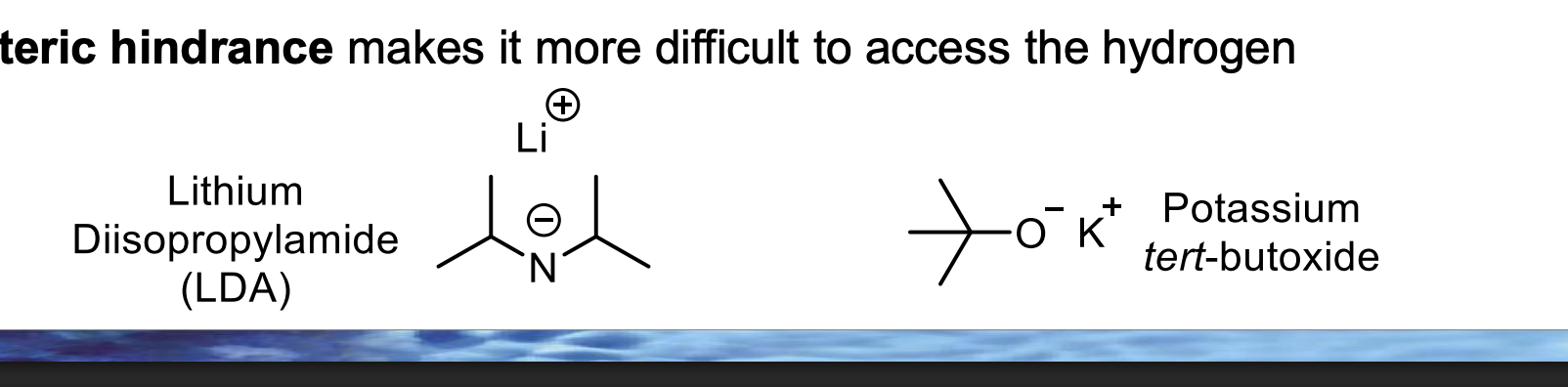
In the BOTTOM diagram, which β-hydrogen does the bulky base actually remove — LEFT or RIGHT?
Because E2 happens in one step, and the base removes the hydrogen it can grab fastest, not the one that would give the most stable alkene.
🧠 Bulky base = speed + access > stability.
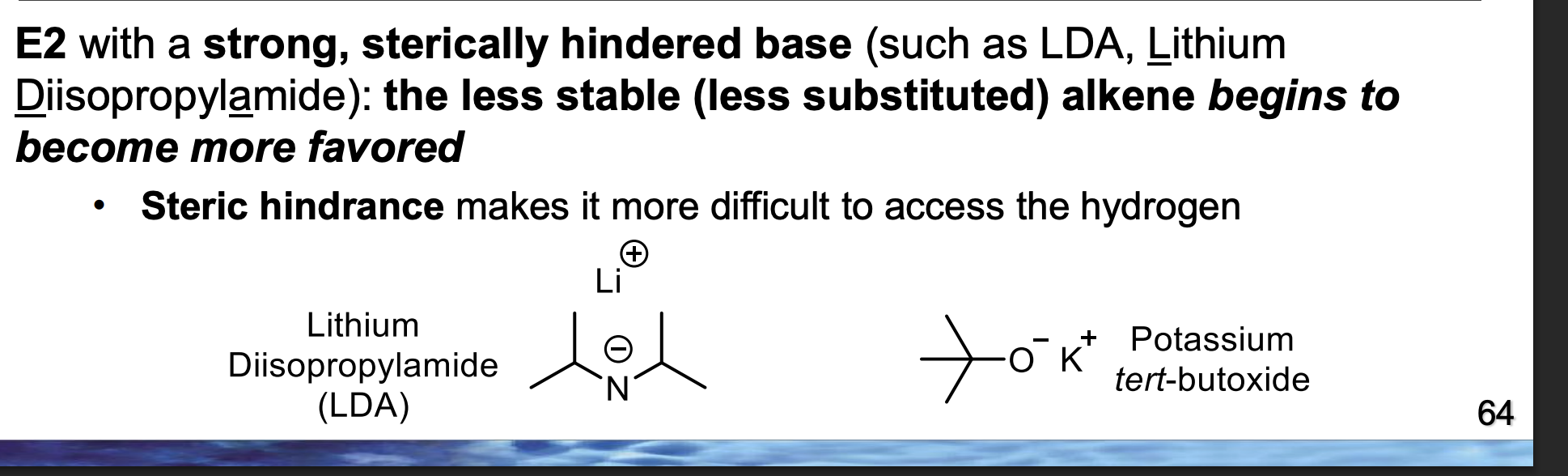
In the BOTTOM diagram, which alkene is the major product — LEFT or RIGHT?
Because the bulky base controls the reaction by steric accessibility, not alkene stability.
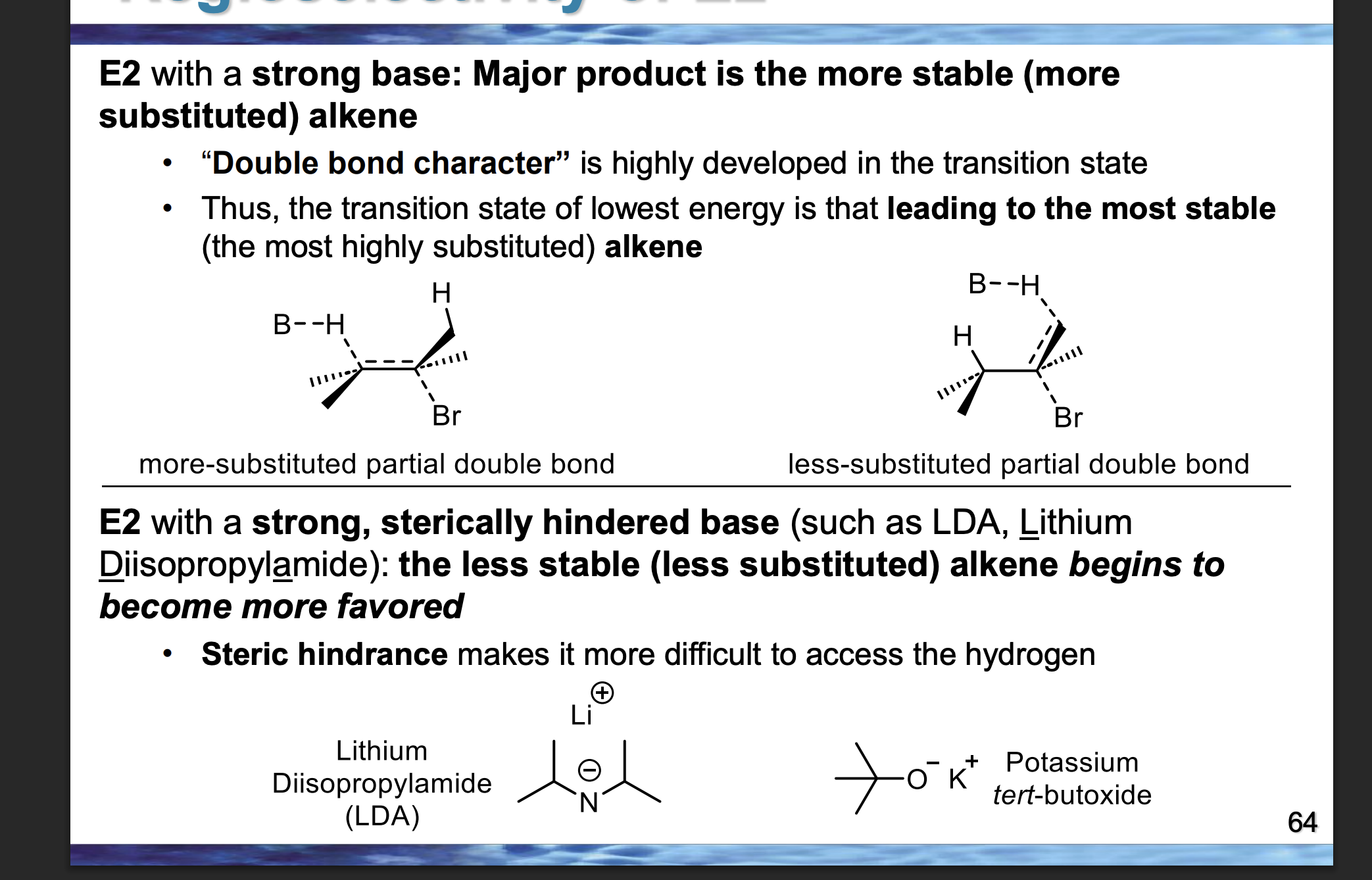
Front (diagram):
Top diagram major product = LEFT
Bottom diagram major product = RIGHT
👉 Why did the preference flip?
Because changing the base from small → bulky changed which β-hydrogen could be reached fastest during the single E2 step.
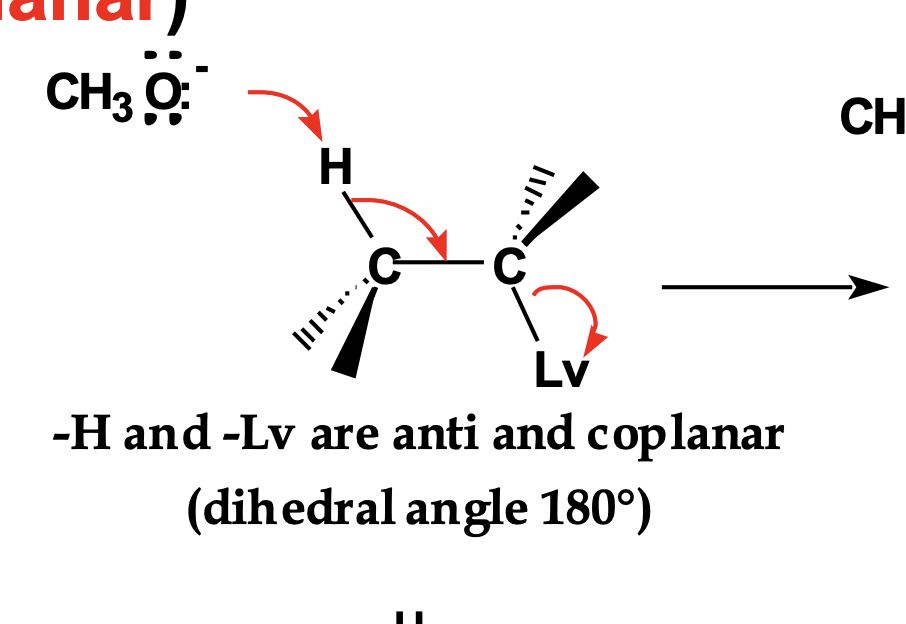
Look at the top reaction drawing with red arrows.
👉 How are the β-H and the leaving group (Lv) positioned relative to each other?
They are anti-coplanar (anti-periplanar) — 180° apart.
This alignment lets the C–H bond electrons overlap perfectly with the C–Lv antibonding orbital, lowering activation energy.
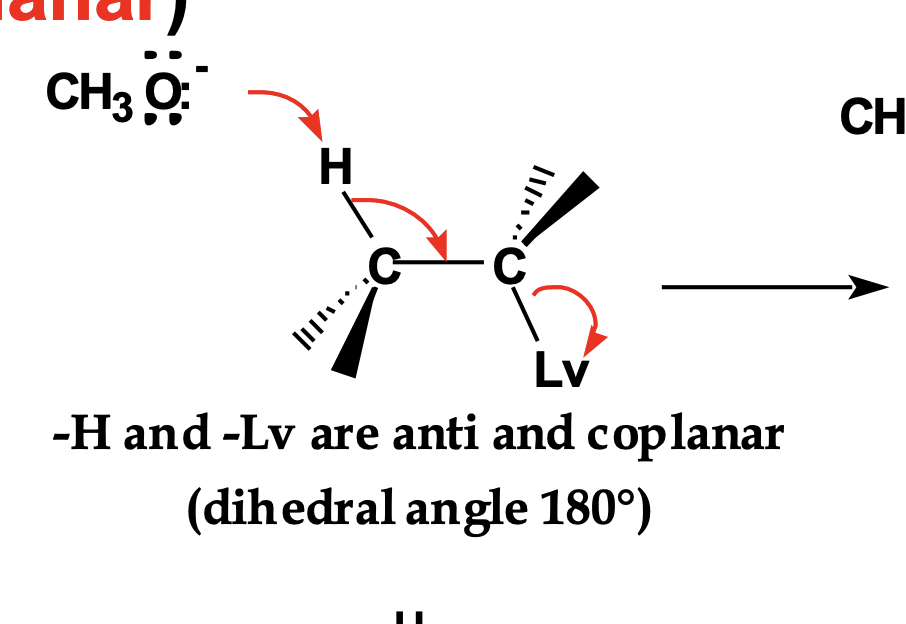
Imagine rotating the molecule so H and Lv are not 180° apart.
👉 What happens to the reaction rate?
The rate drops sharply or the reaction does not occur, because poor overlap raises the activation energy.
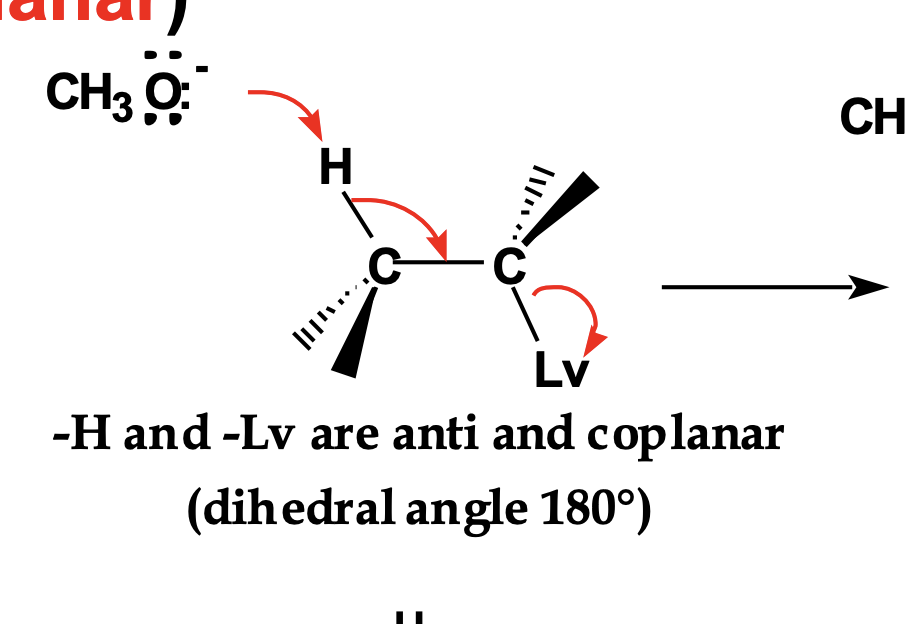
In the top structure, there are multiple β-hydrogens.
👉 Which β-hydrogen can react in E2?
Only the β-hydrogen that is anti-periplanar to the leaving group.
Other hydrogens are geometrically blocked, even if they would give a stable alkene.
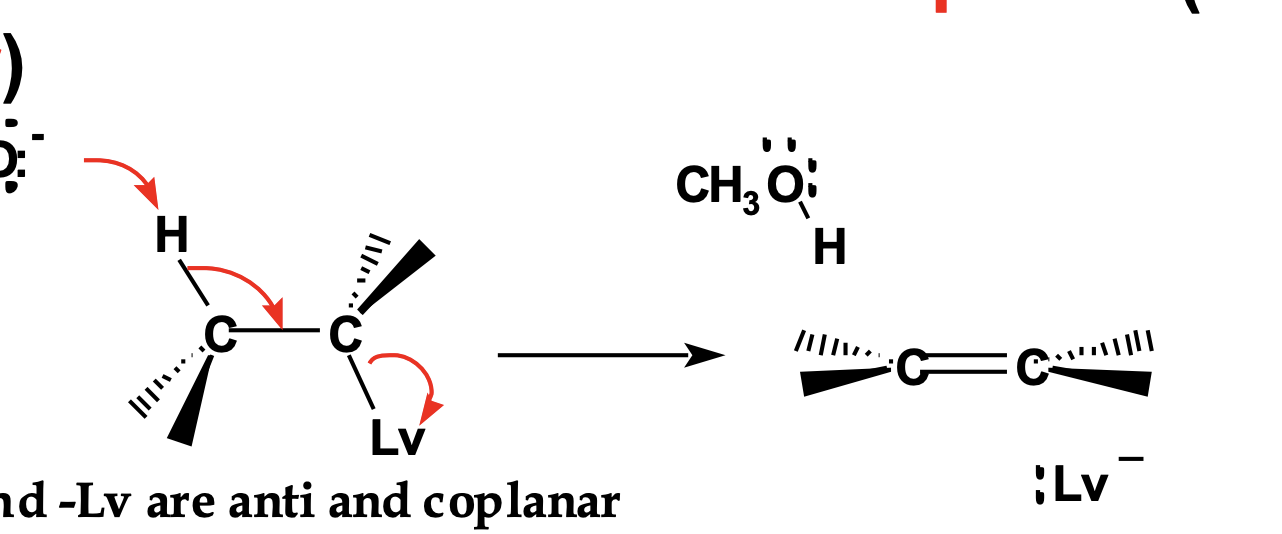
draw the newman projection
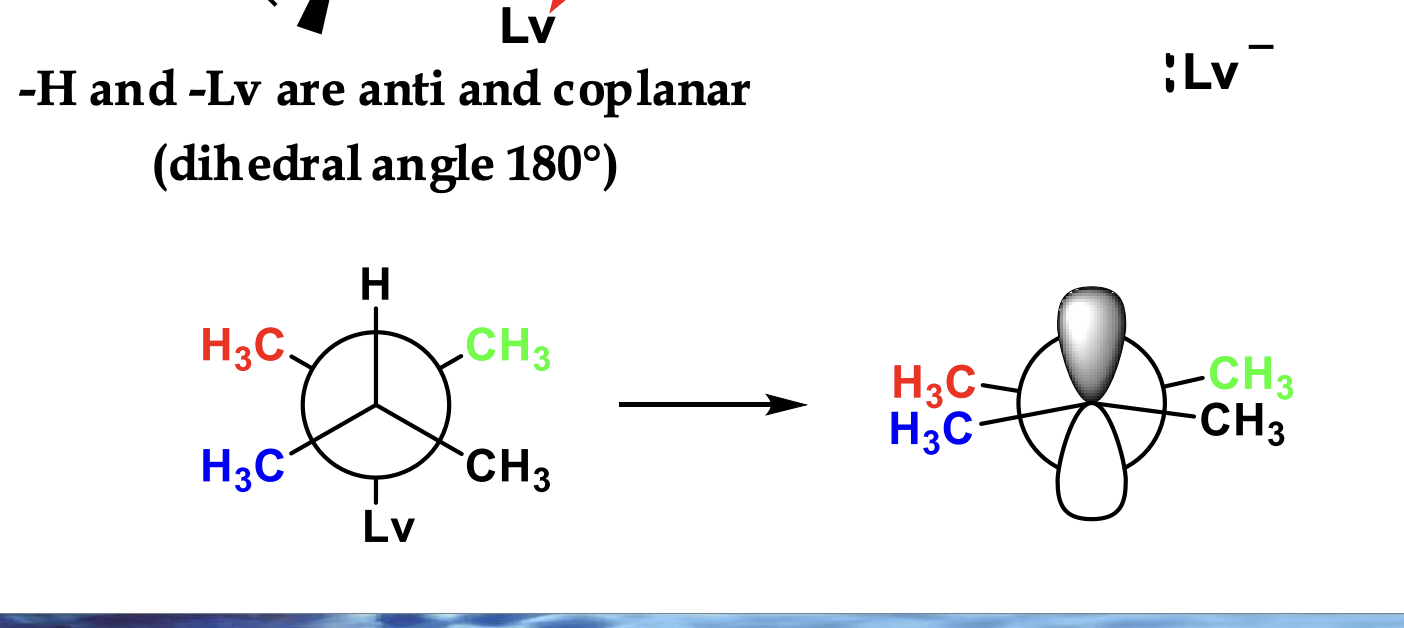
E2 ONLY works when the leaving group (Cl) is AXIAL. (true/f)
true
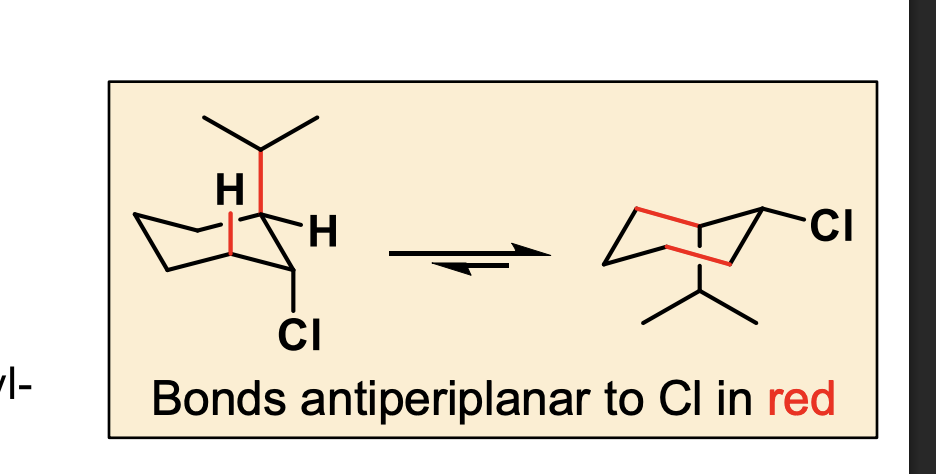
which out of these two will react?
Chair B (after ring flip)
Cl is axial
✅ NOW E2 is possible
Because there IS an axial β-H anti to Cl
👉 This chair is the one that reacts
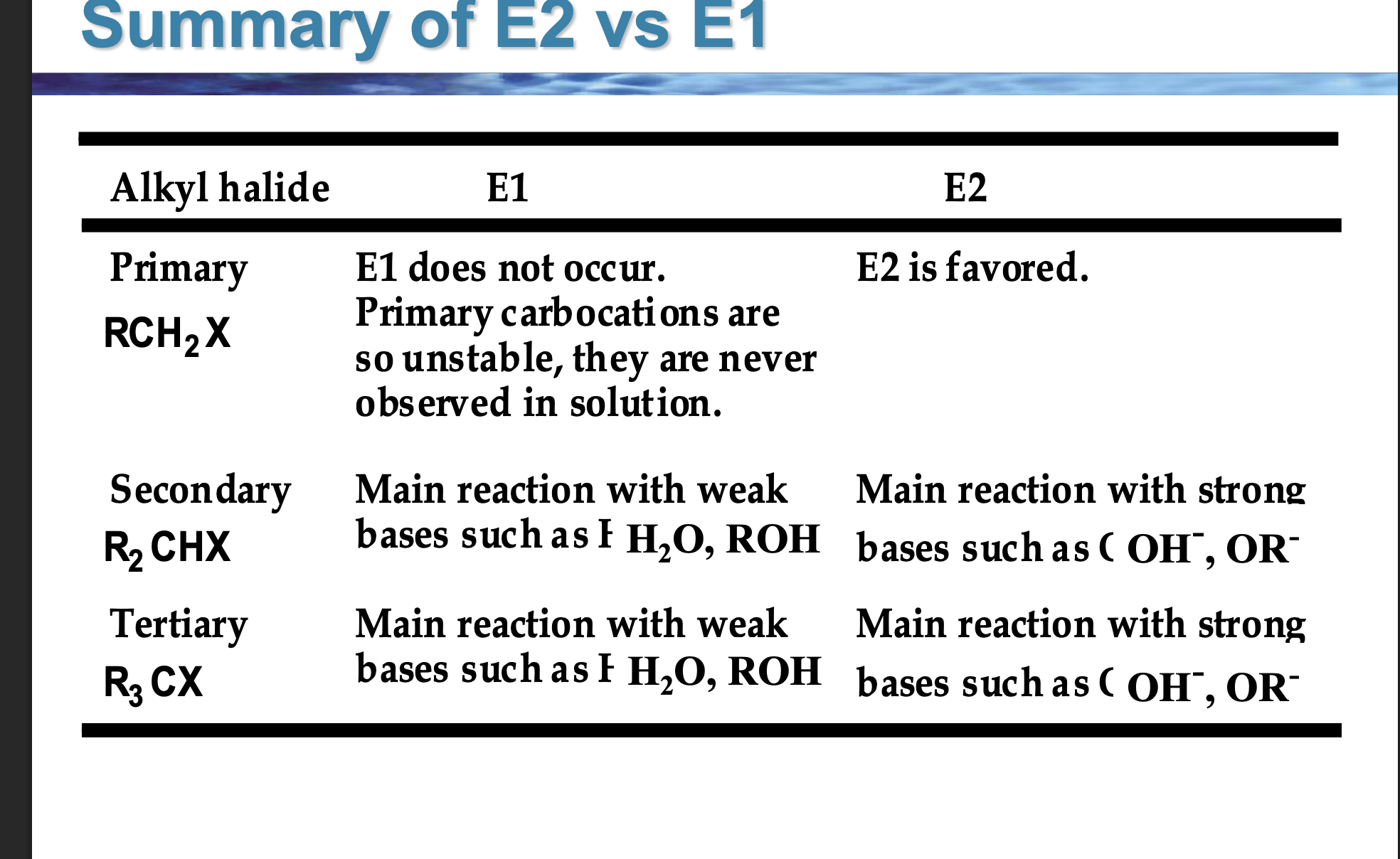
👉 Does E1 occur for primary alkyl halides?
E1 requires a carbocation, and primary carbocations are extremely unstable, so they do not form in solution.
If you see RCH₂X, what elimination mechanism should you immediately think?
E2 only.
(E1 is off the table.)
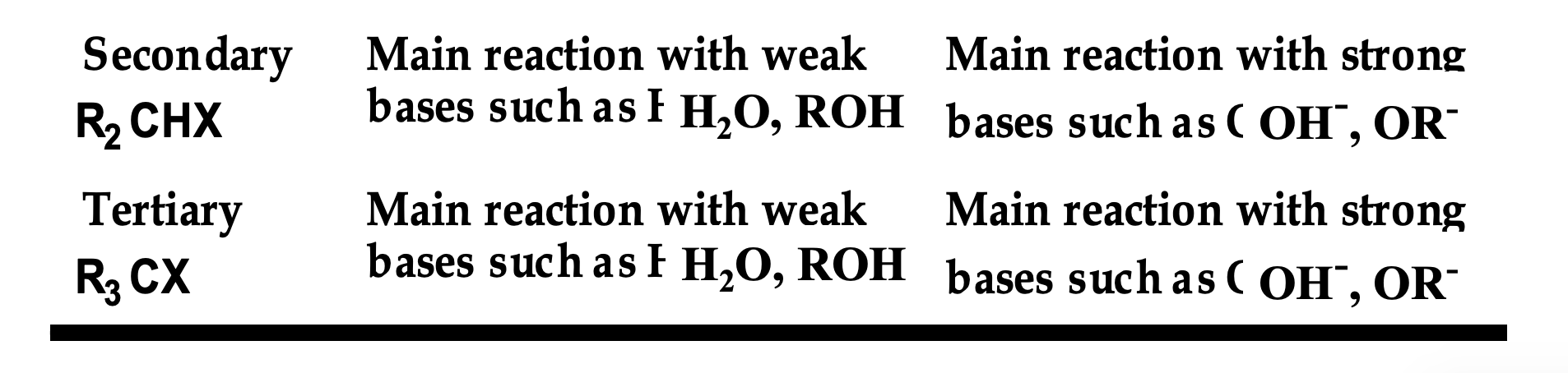
Front (table):
Look at the SECONDARY + weak base (H₂O, ROH) entry.
👉 Which mechanism dominates?
Back (WHY):
E1.
Weak bases cannot pull off a β-H in one step, so the reaction proceeds by carbocation formation first.
Complete this rule:
Primary → ______
Secondary → depends on ______
Tertiary → depends on ______
Back:
Primary → E2
Secondary → base strength
Tertiary → base strength
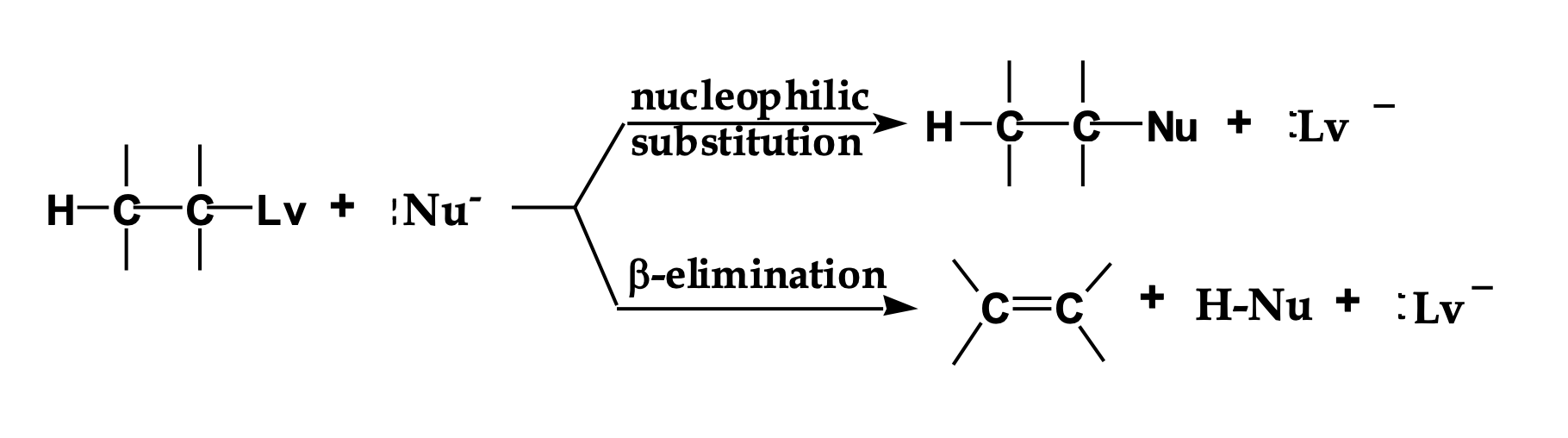
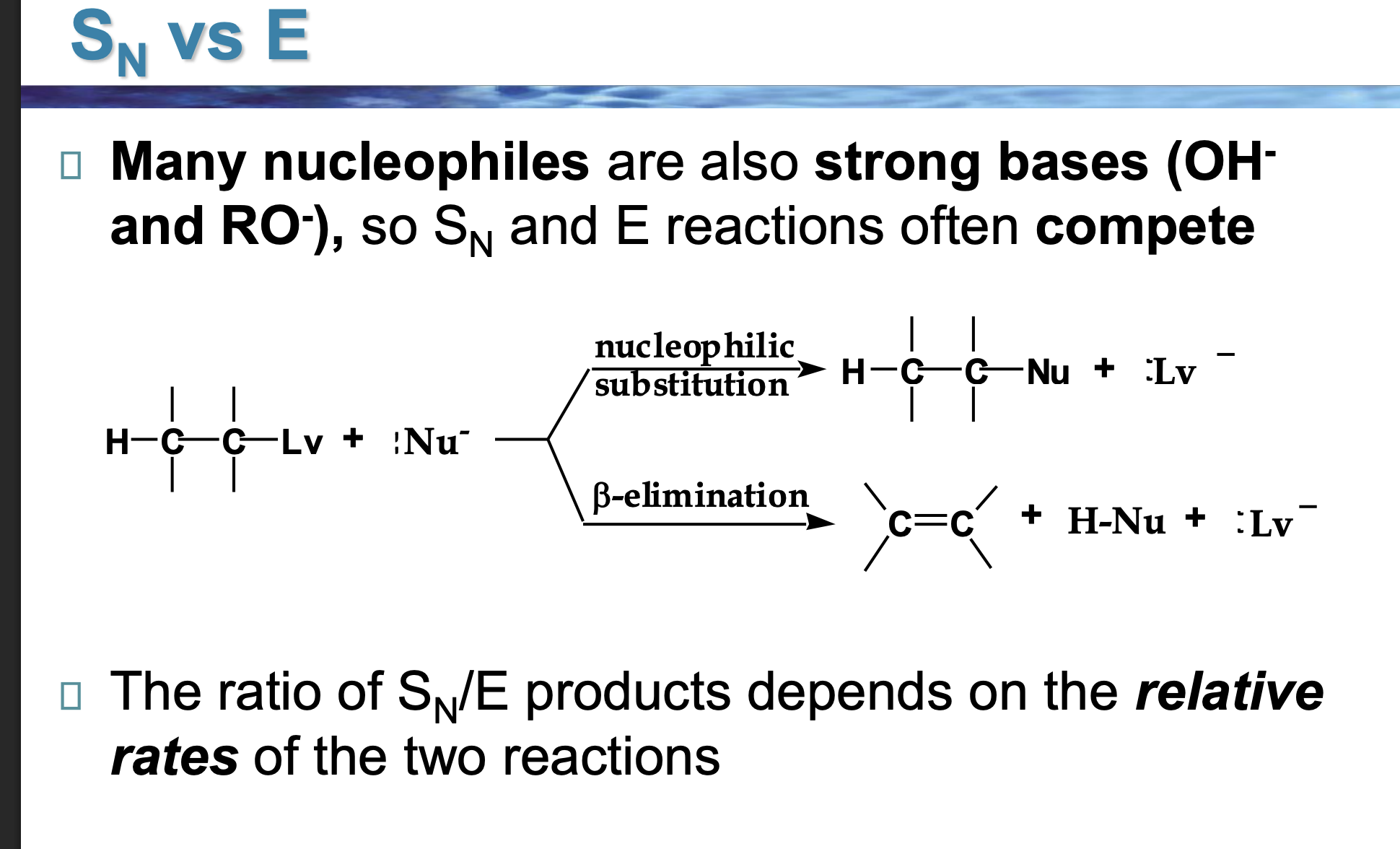
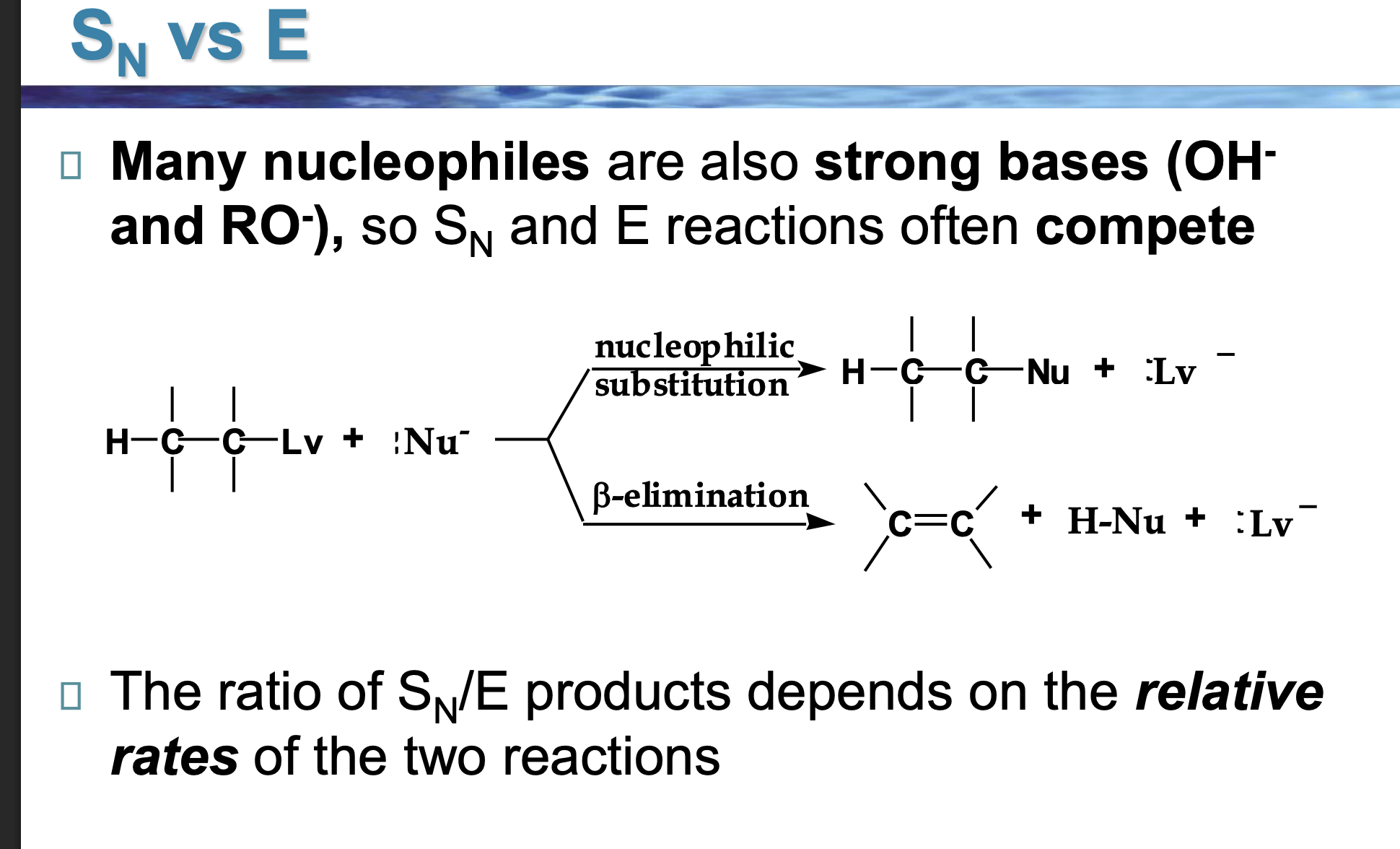
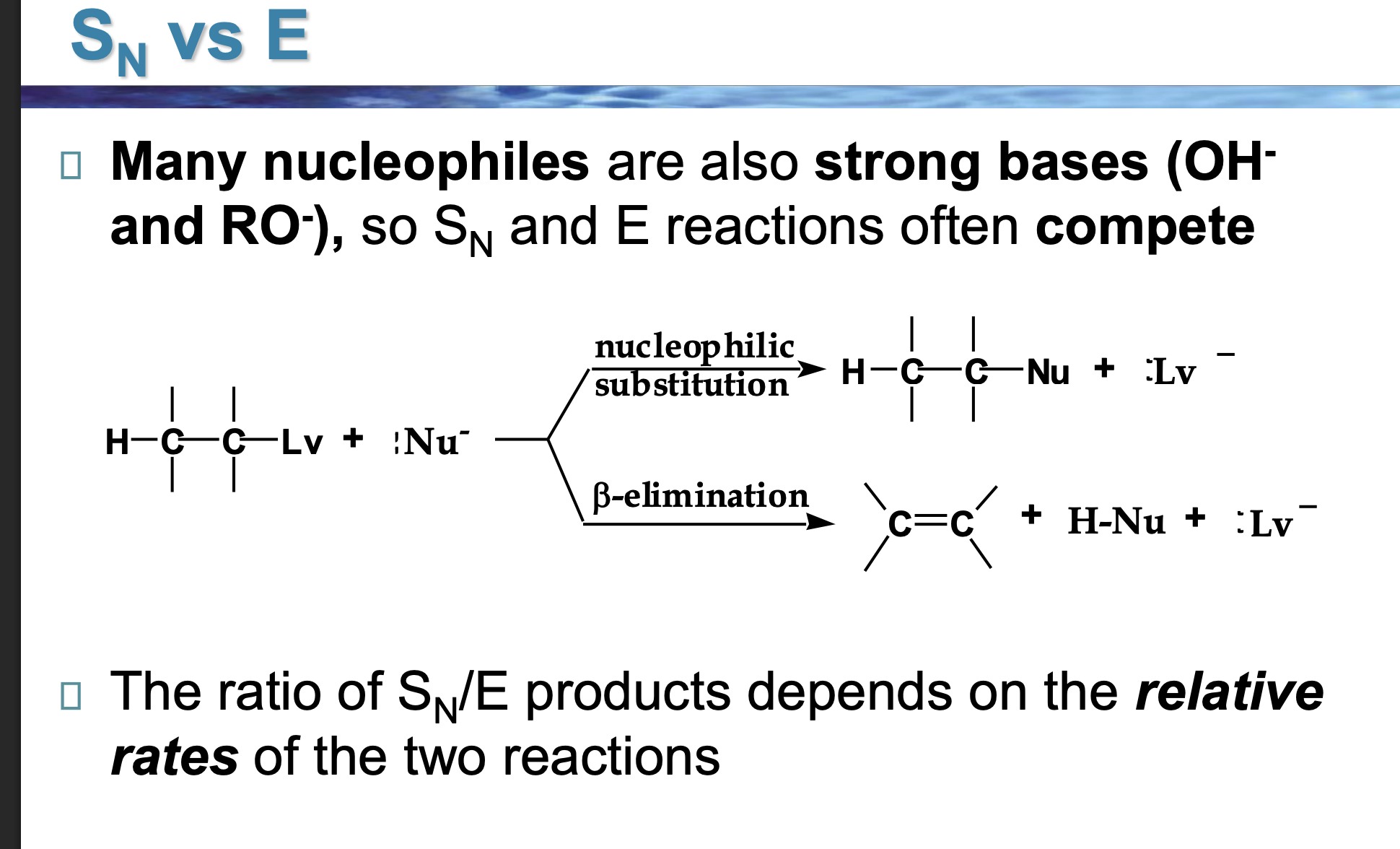
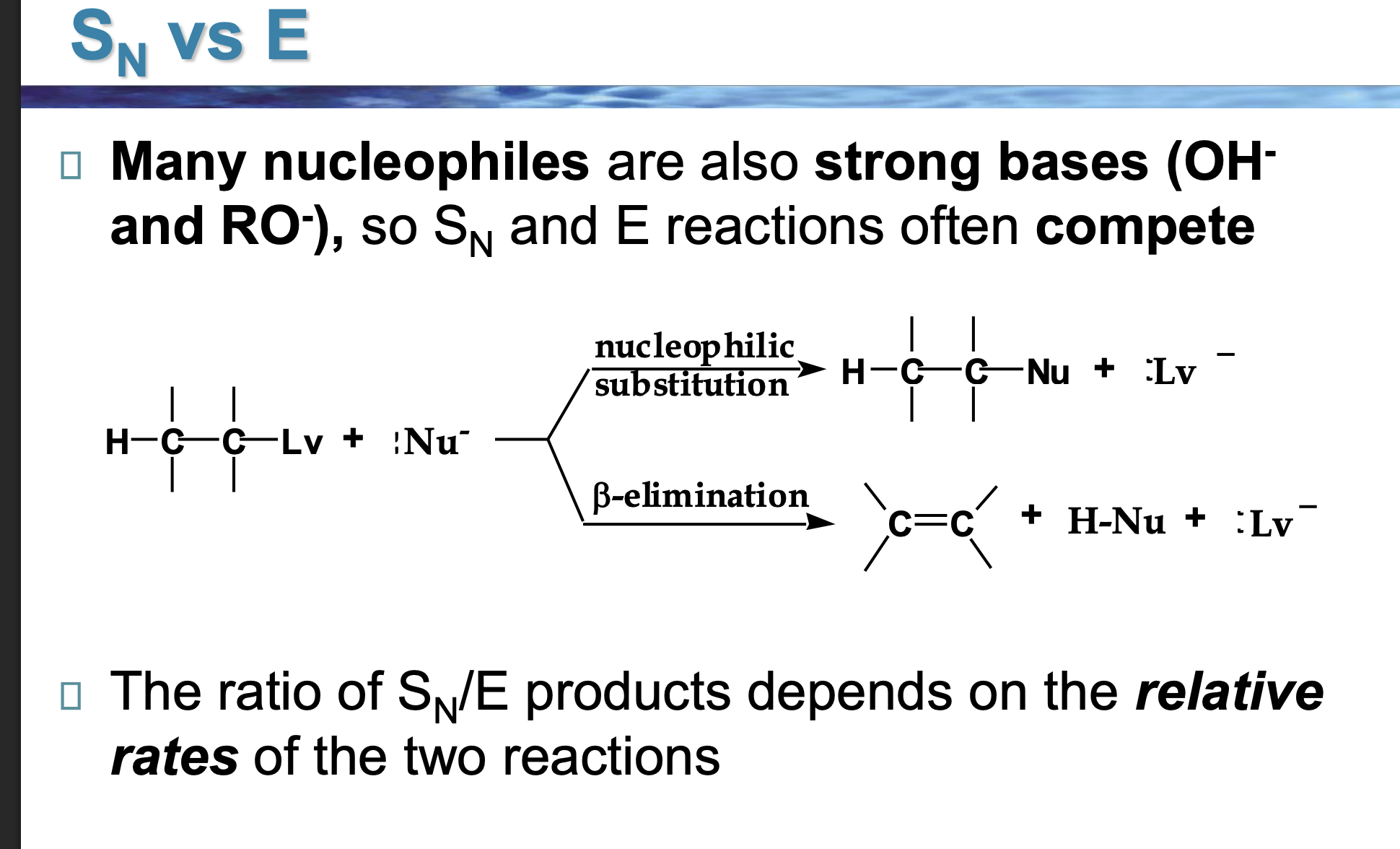
Look at the LEFT side of the diagram.
👉 What two things are reacting?
An alkyl halide (carbon with Lv attached)
A Nu⁻ (something with a negative charge)
This Nu⁻ can do two different things.
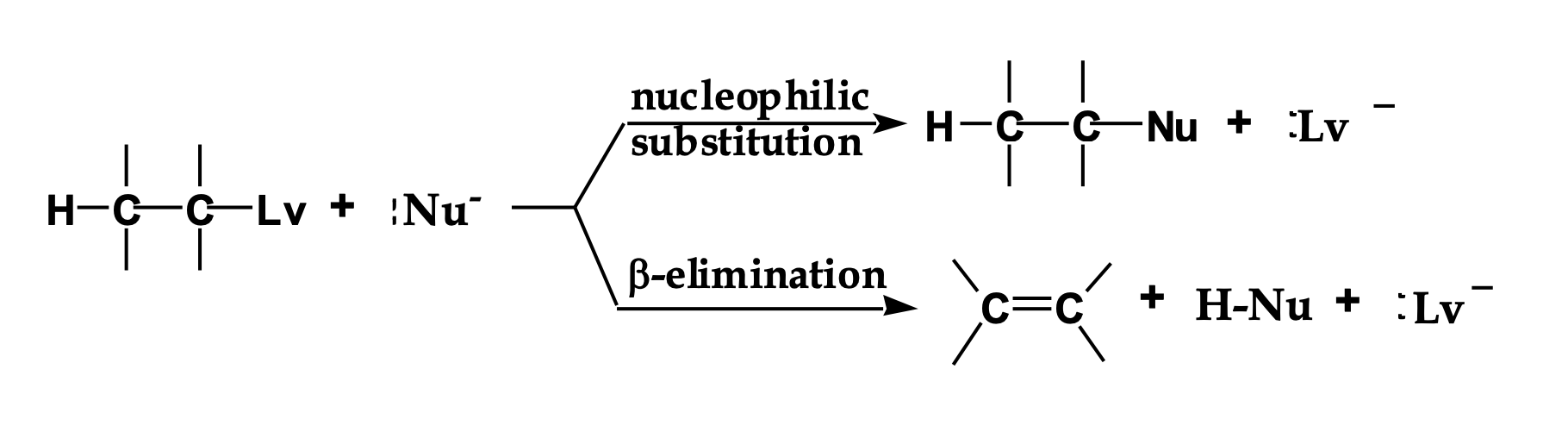
The reaction splits into two arrows.
👉 Why does it split?
Because Nu⁻ has two choices:
Go for the carbon → substitution
Go for a hydrogen → elimination
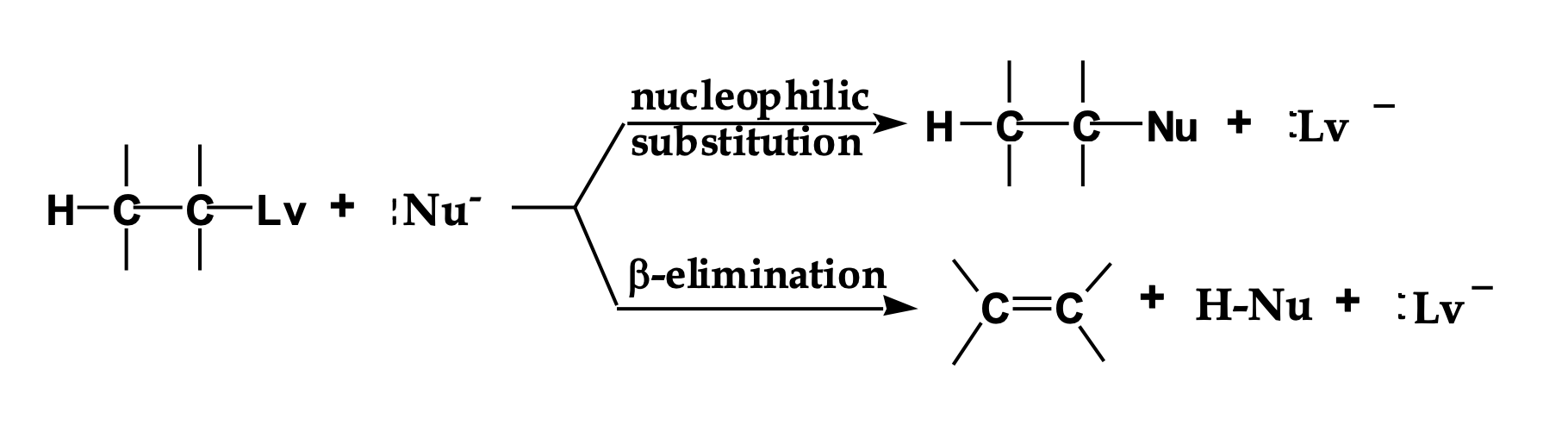
Look at the top pathway.
👉 What changes in the molecule?
The nucleophile replaces the leaving group.
Nothing is removed — one thing is swapped.

Look at the bottom pathway.
👉 What changes in the molecule?
A hydrogen and the leaving group are removed, and a double bond forms.
That’s elimination.
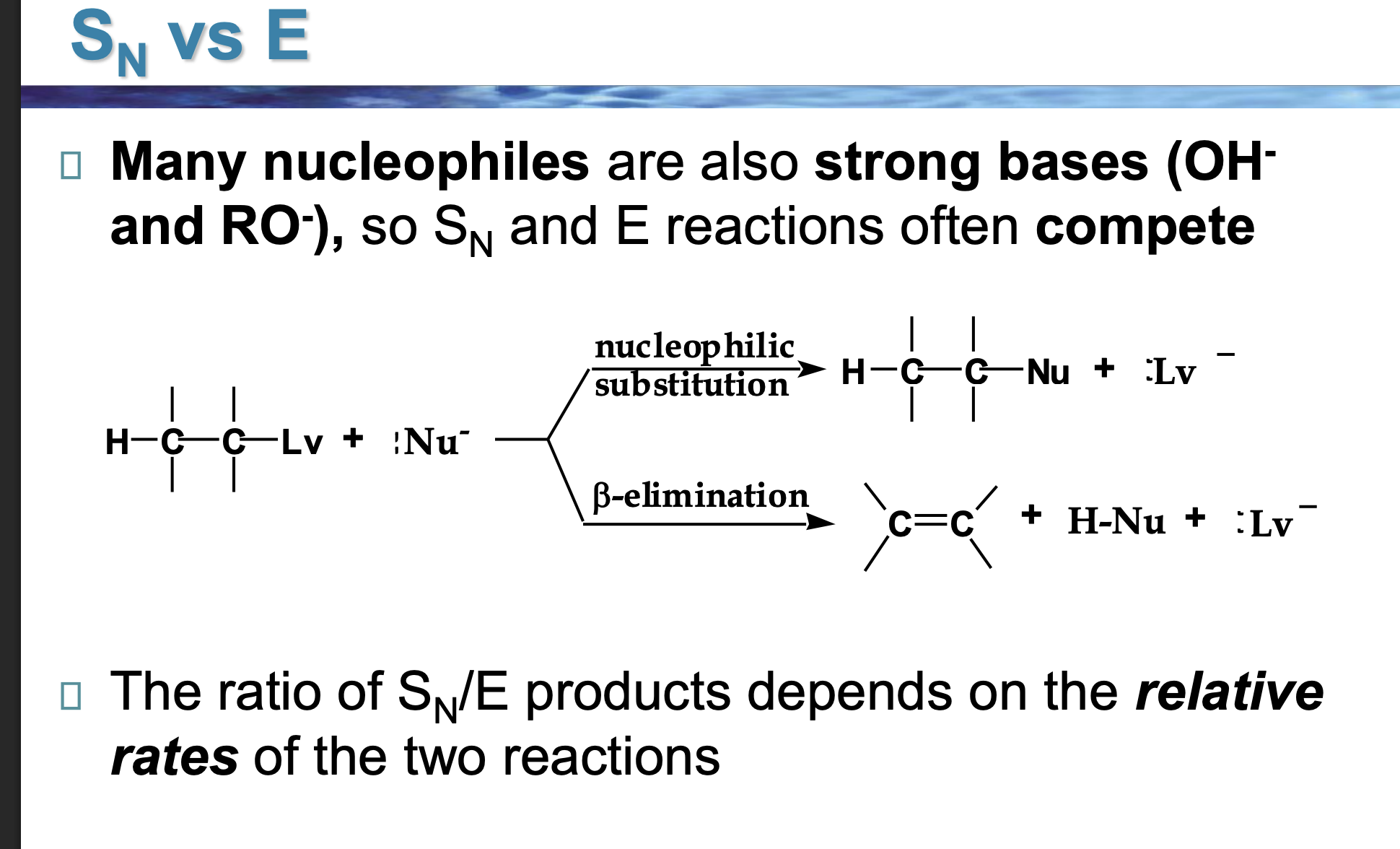
Nu⁻ is shown in both reactions.
👉 What TWO jobs can it do?
As a nucleophile → attacks carbon (Sᴺ)
As a base → removes hydrogen (E)
Same species. Different behavior.
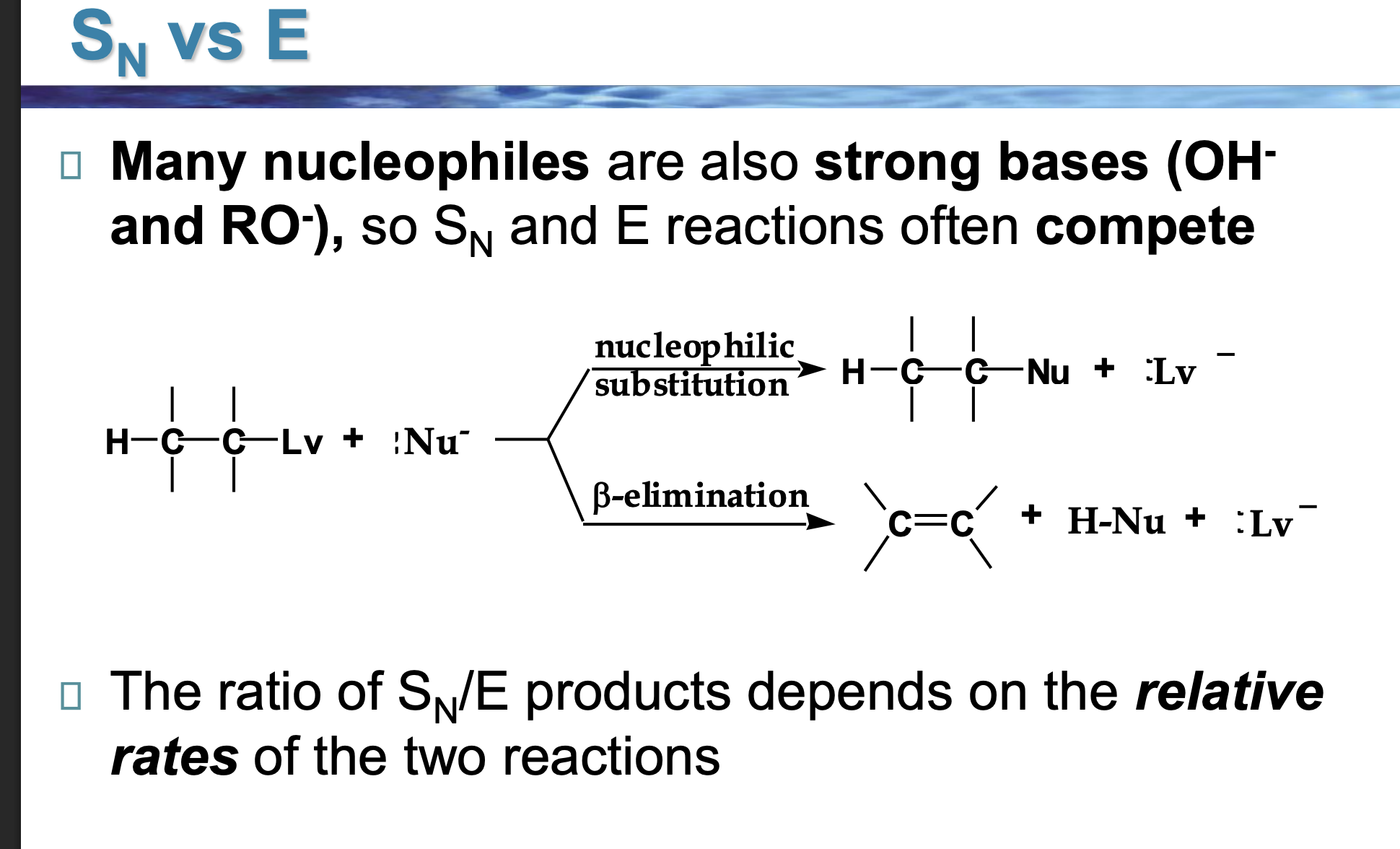
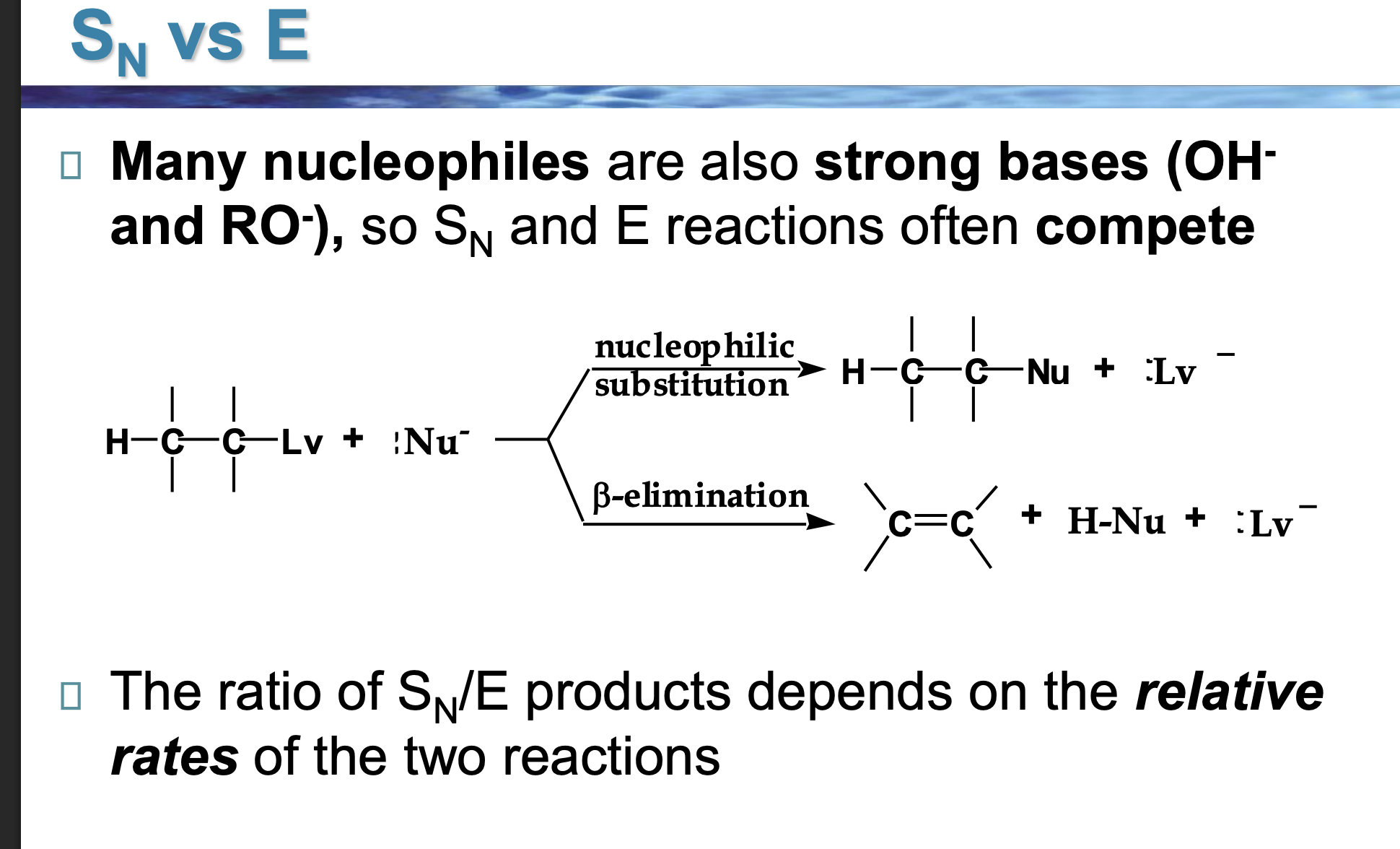
Why do OH⁻ and RO⁻ cause competition?
Because they are:
Good at attacking carbon
ALSO good at grabbing hydrogens
So Sᴺ and E happen at the same time.
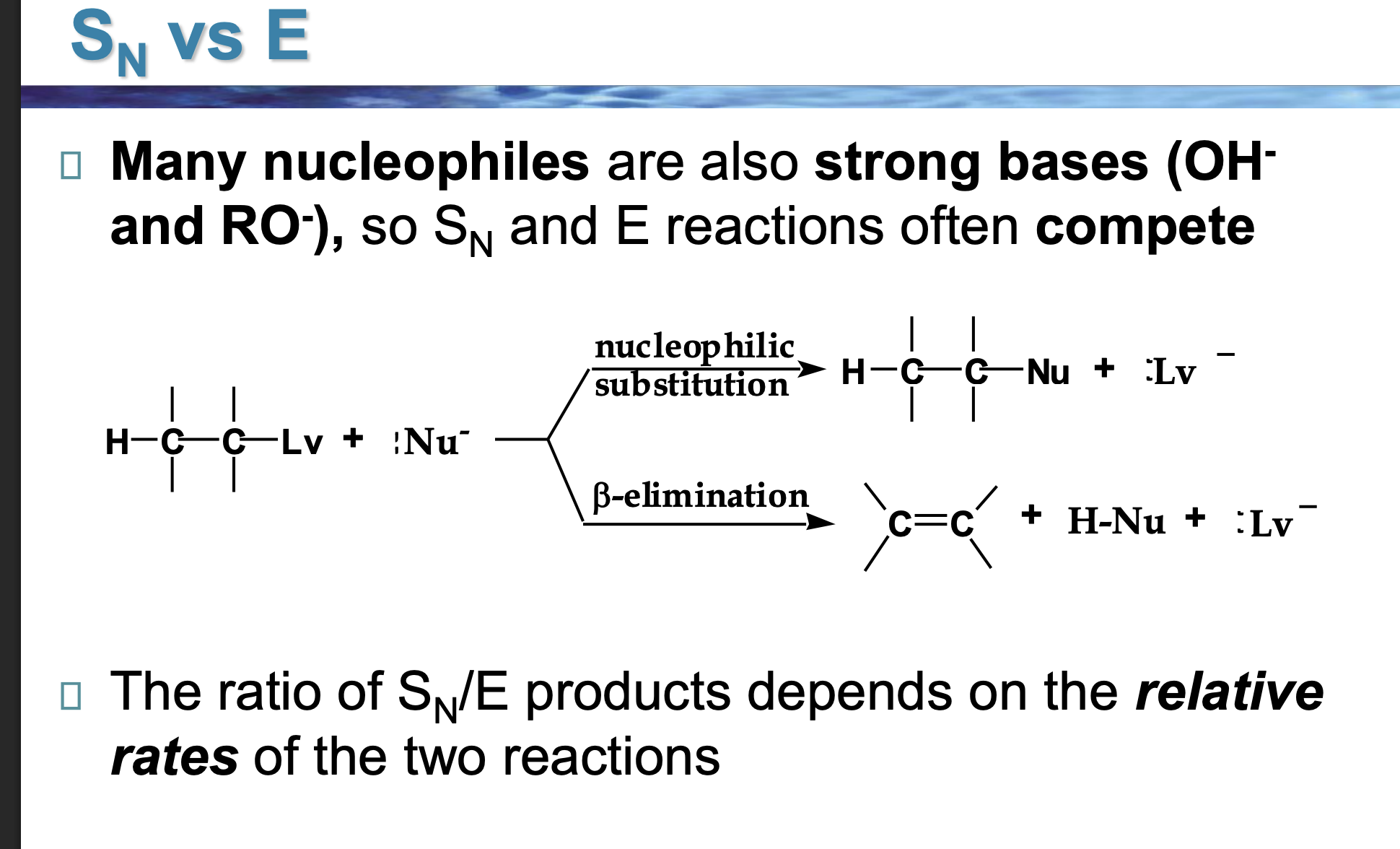
Does only ONE reaction happen?
Back (WHY):
No.
Both happen, but one happens more, so it gives more product.
The slide says “relative rates.”
👉 What does that mean in normal words?
Back (WHY):
Whichever reaction happens faster makes more product.
It’s a race.
Front:
If Nu⁻ is a strong base, which path speeds up?
Back (WHY):
Elimination.
Strong bases are really good at grabbing hydrogens.
Card 10 — Heat effect
Front:
If the reaction is heated, which path is helped?
Back (WHY):
Elimination.
Elimination makes more pieces → heat favors that.
Card 11 — Weak base, good nucleophile
Front:
If Nu⁻ is a weak base but good nucleophile, which path wins?
Back (WHY):
Substitution.
It prefers attacking carbon over removing hydrogen.
Methyl and primary halides never do Sᴺ1 or E1 because they can’t form carbocations.
Primary halides do Sᴺ2 with good nucleophiles,
and E2 only when the base is strong and bulky.
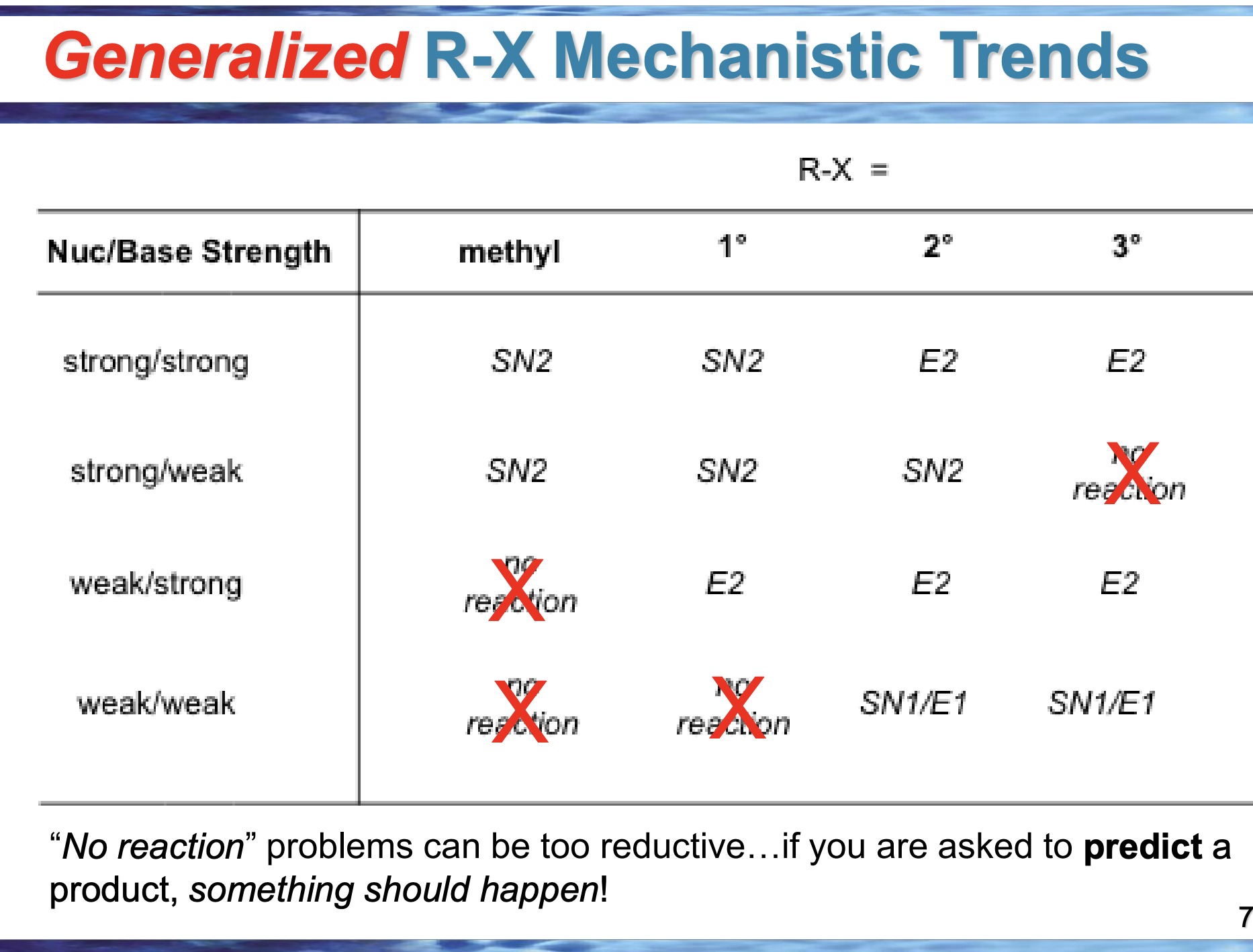
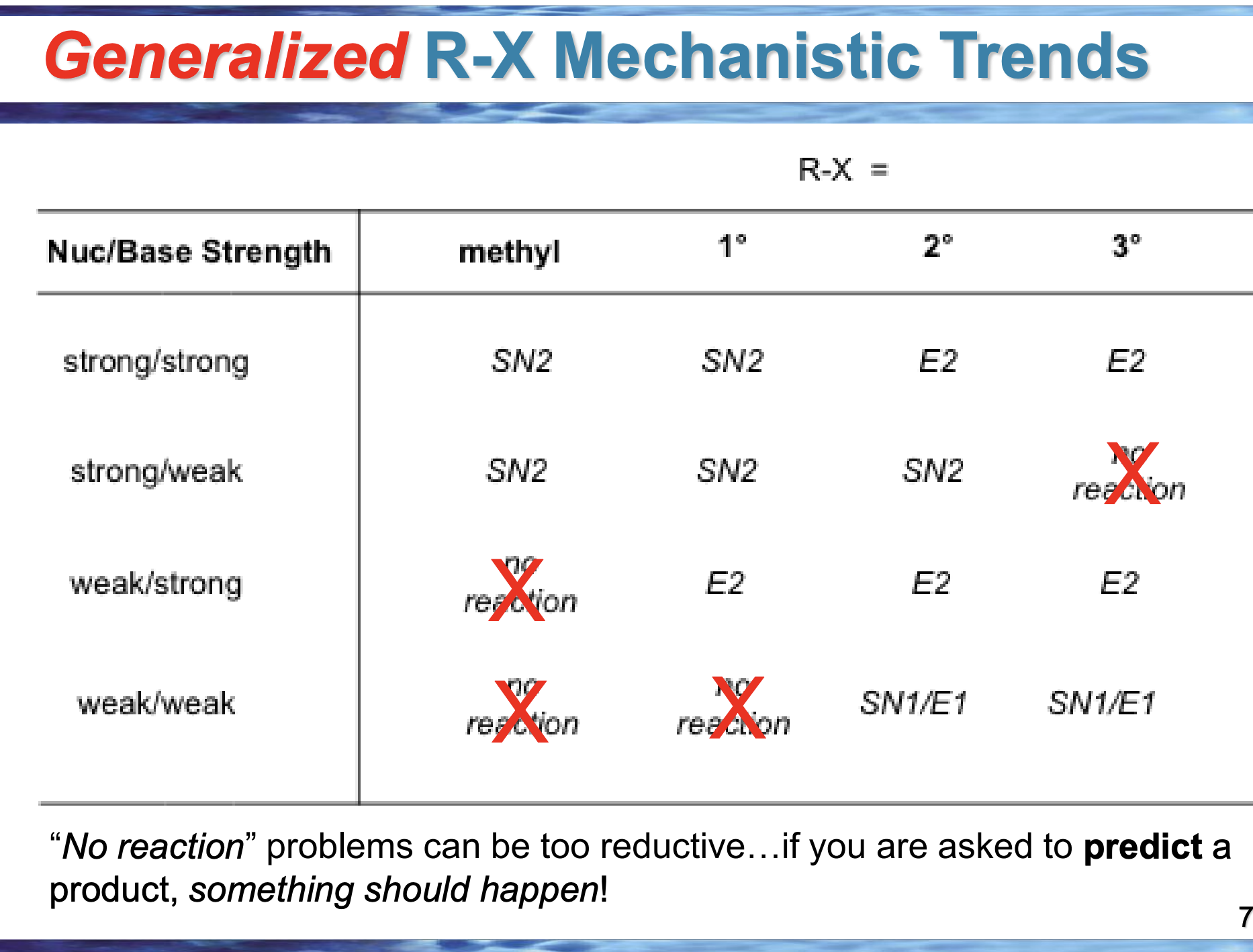
If nothing special is done, which reaction usually wins: Sᴺ1 or E1?
Sᴺ1, because nucleophile attack is easier than elimination
What symbol on the slide tells you E1 is being favored?
Δ (heat)
Why does having a poor (or no) nucleophile push toward E1?
Because the carbocation can’t be attacked, so it loses H instead
If the product has a double bond, which pathway happened?
Back:
E1