Science Exam Revision Semester 2 - Chemical Reactions
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
Reactants
A starting material in a chemical reaction
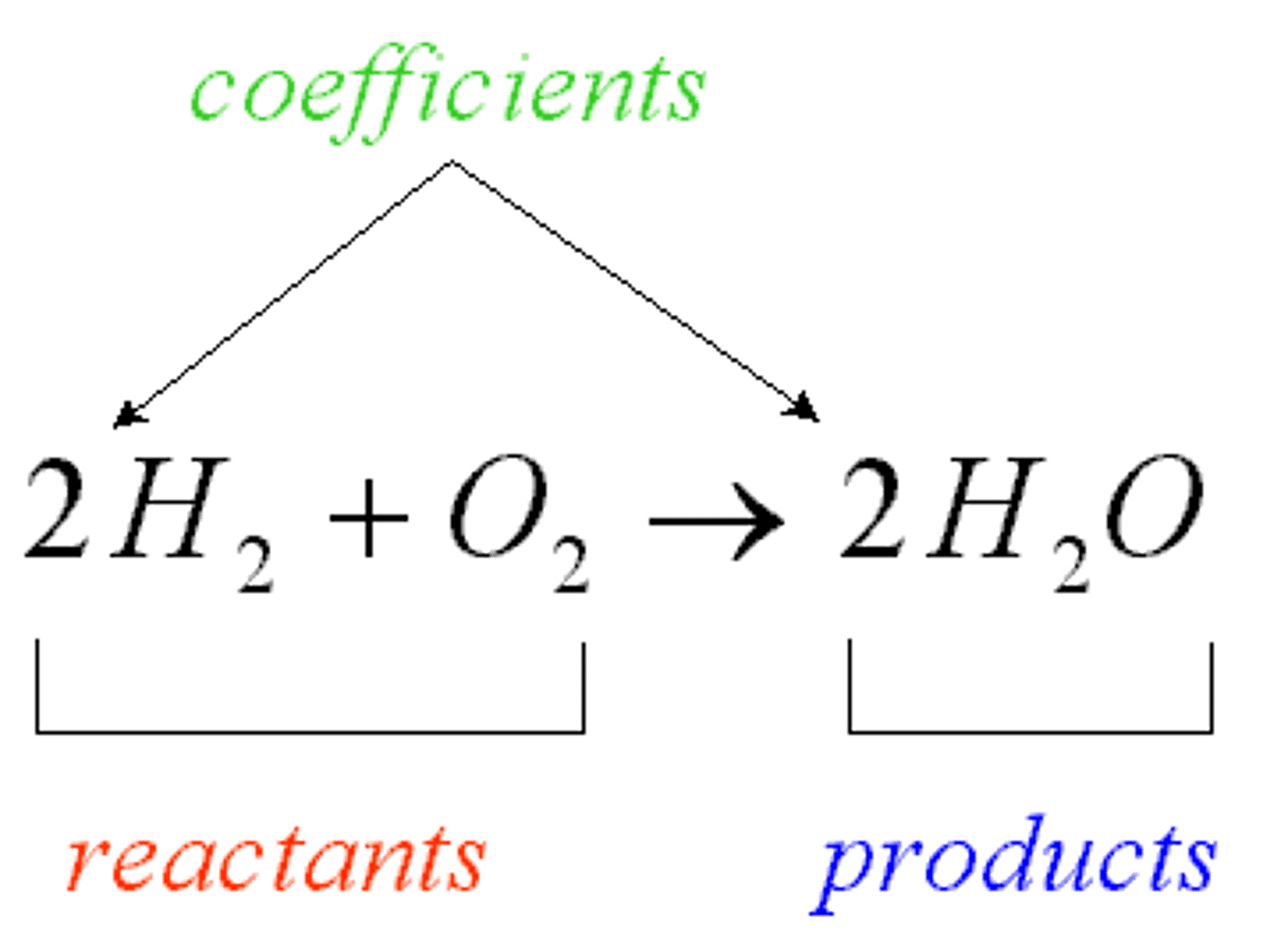
Products
The elements or compounds produced by a chemical reaction.
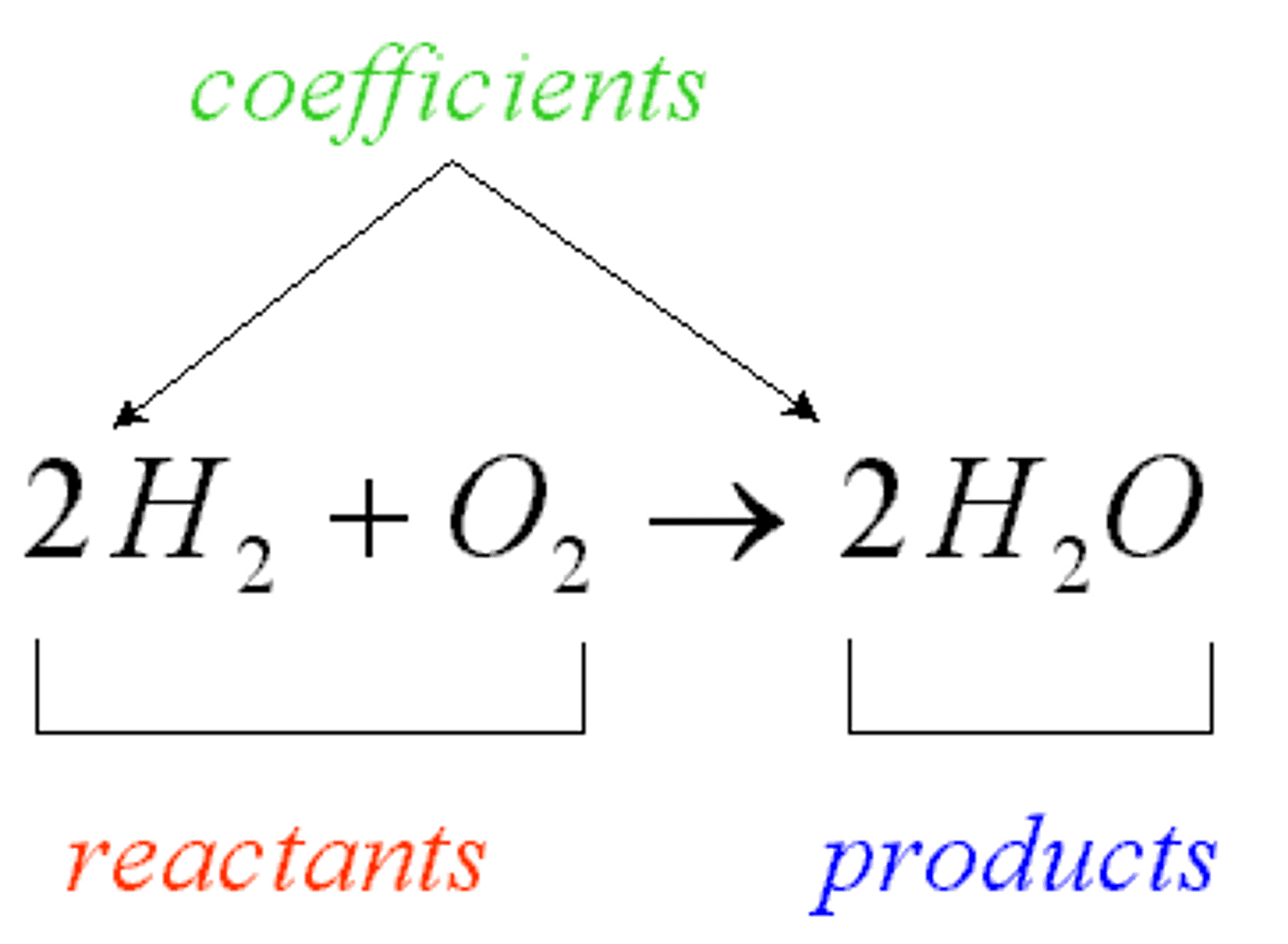
Chemical Reaction
the process by which one or more substances change to produce one or more different substances

Chemical change
A change in matter that produces one or more new substances

Law of Conservation of Mass
Matter is not created nor destroyed in any chemical or physical change

Chemical Formula
A combination of chemical symbols and numbers to represent a substance
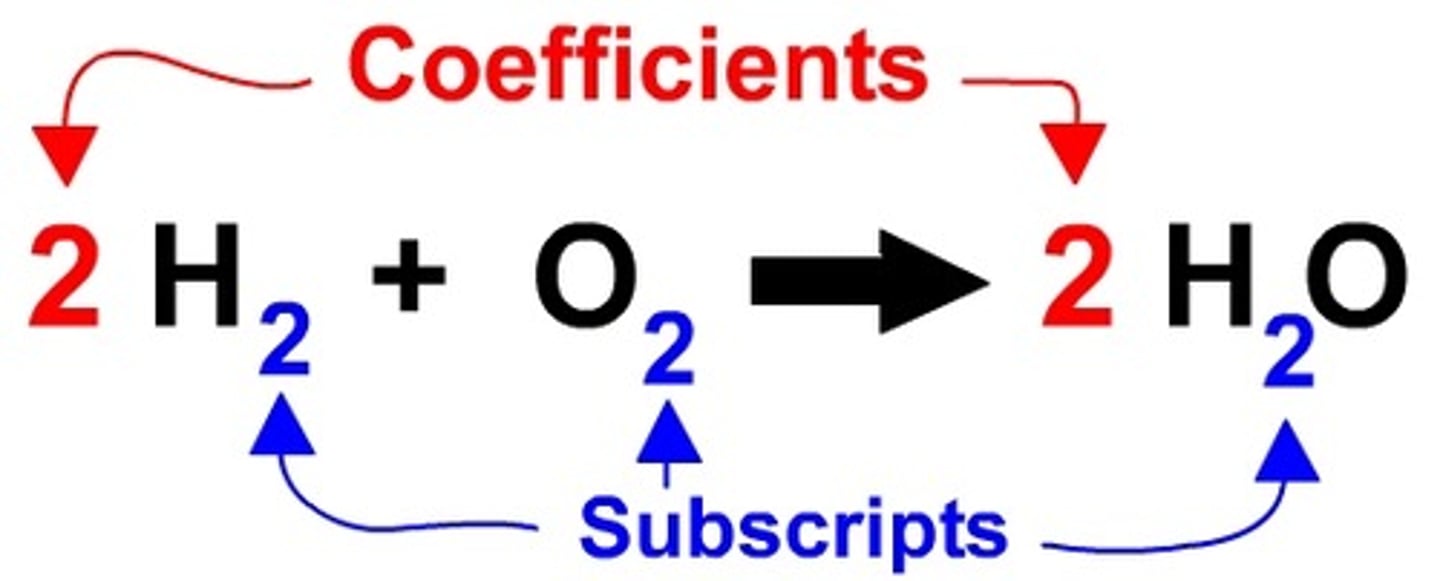
Coefficient
A number in front of a chemical formula in an equation that indicates how many molecules or atoms of each reactant and product are involved in a reaction.
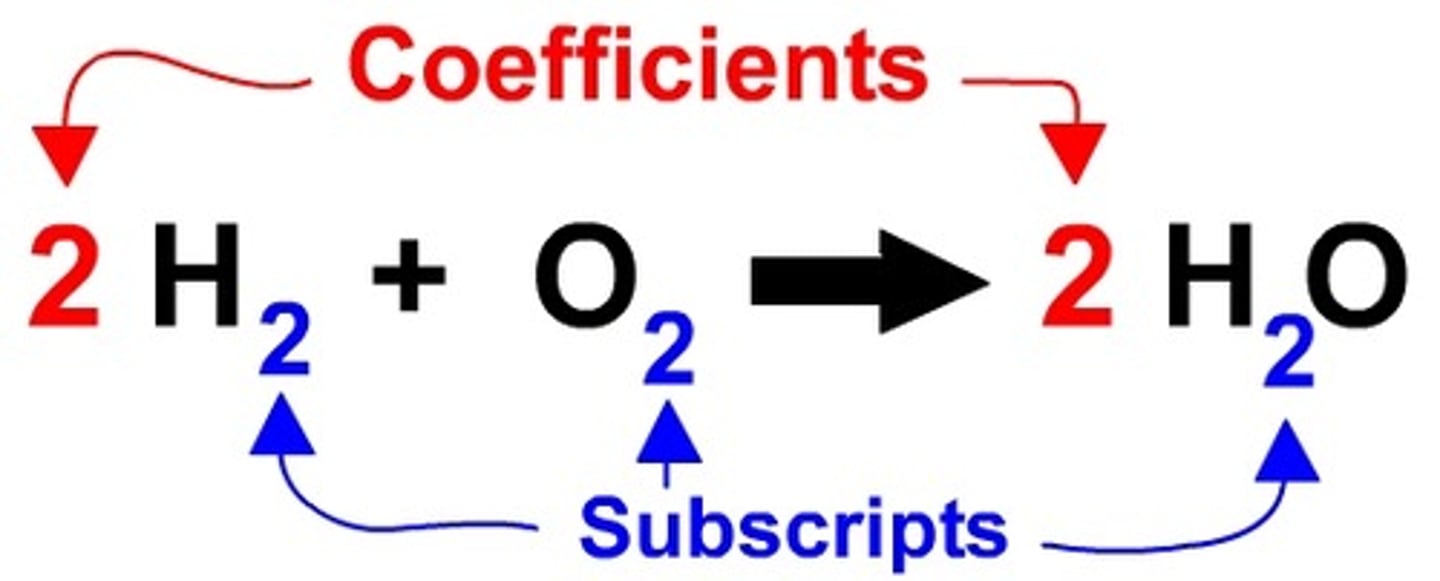
Subscript
A number in a chemical formula that tells the number of atoms in a molecule or the ratio of elements in a compound
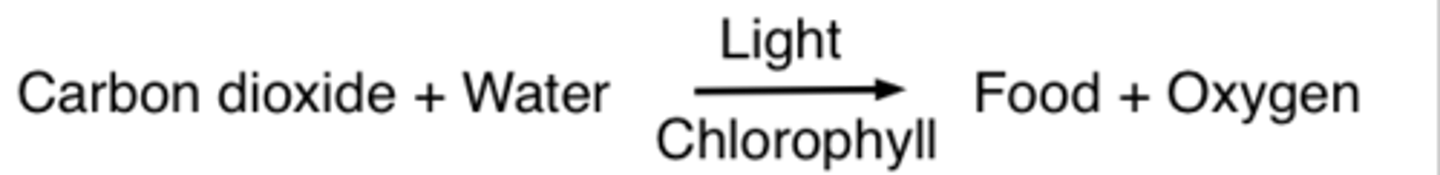
word equation
an equation in which the reactants and products in a chemical reaction are represented by words
Chemical Equation
A representation of a chemical reaction that uses symbols to show the relationship between the reactants and the products
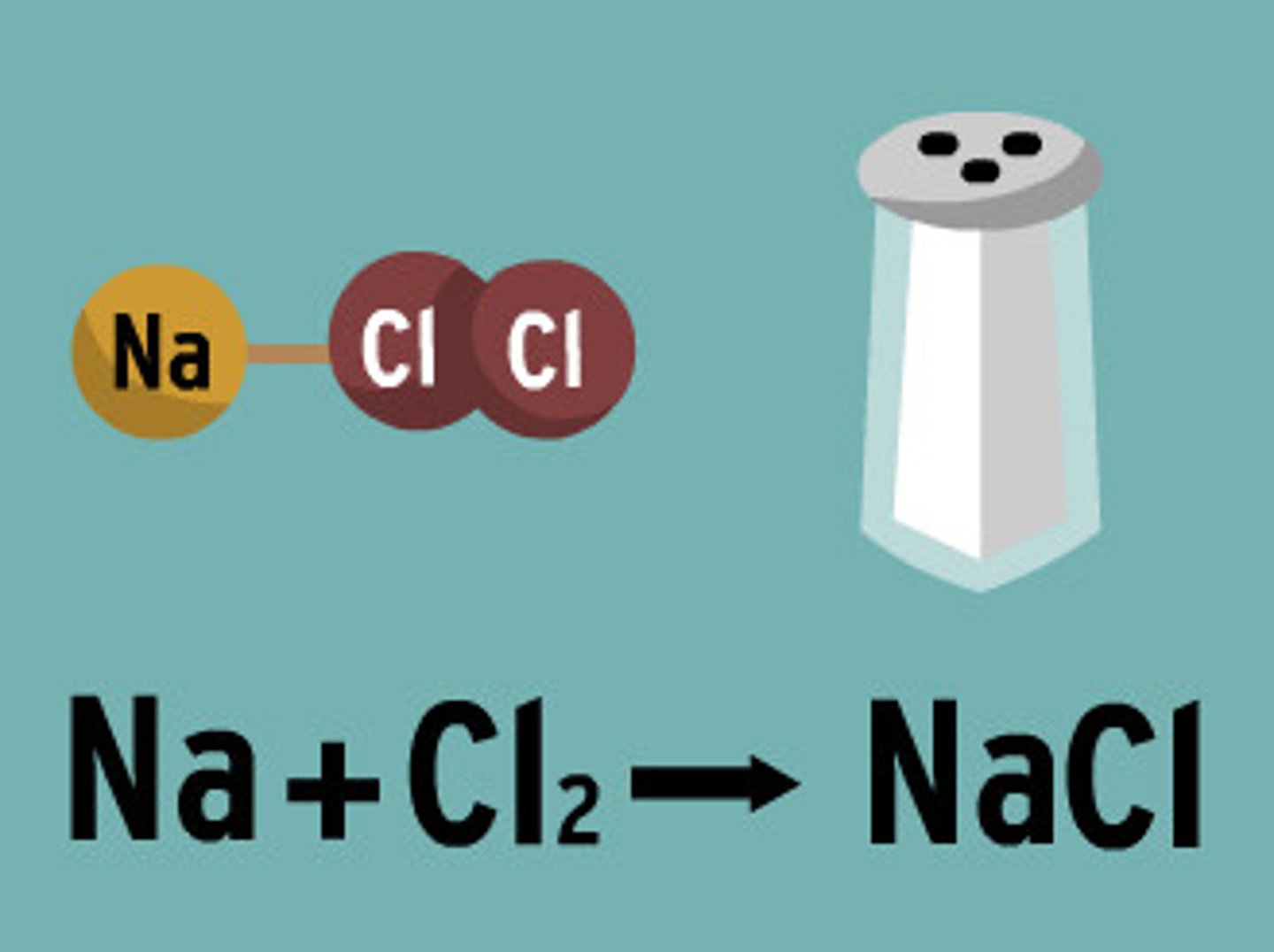
balanced chemical equation
chemical equation with the same number of atoms of each element on both sides of the equation
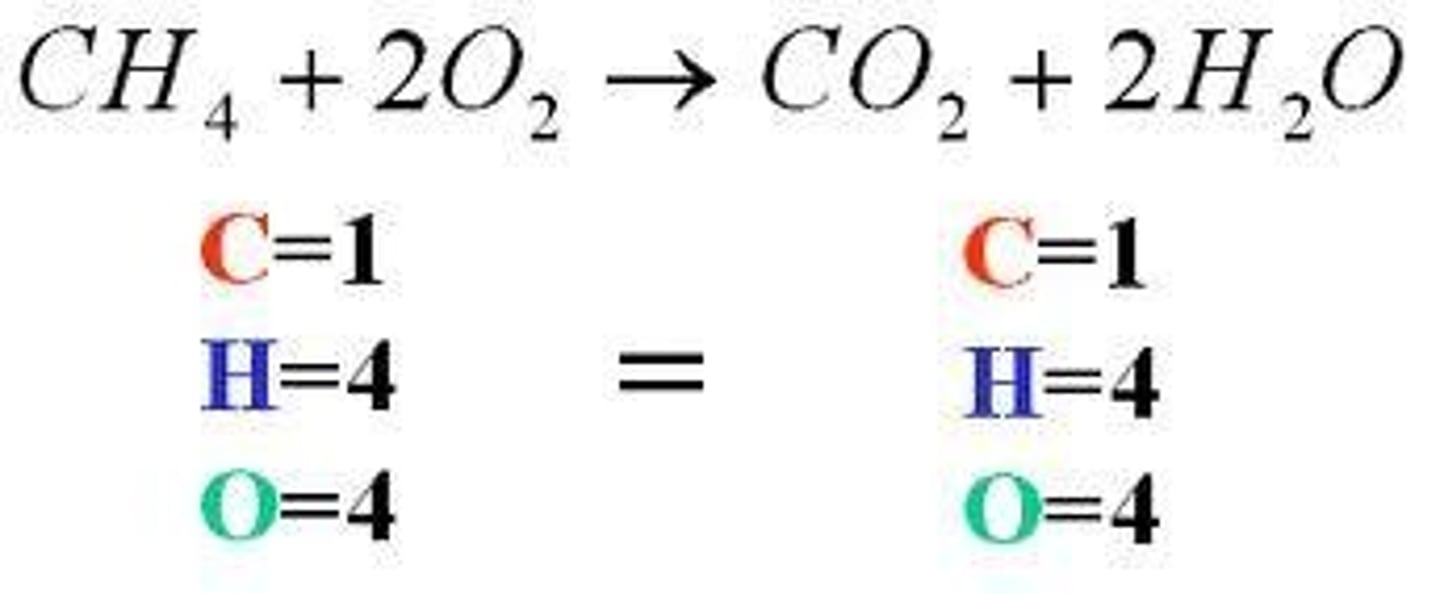
endothermic reaction
A reaction that ABSORBS energy in the form of heat

exothermic reaction
A reaction that releases energy in the form of heat

decomposition reaction
a reaction in which a single compound breaks down to form two or more simpler substances
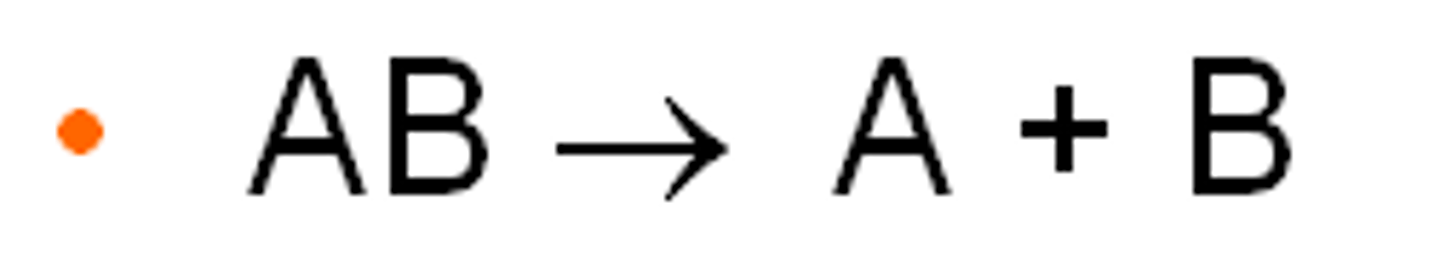
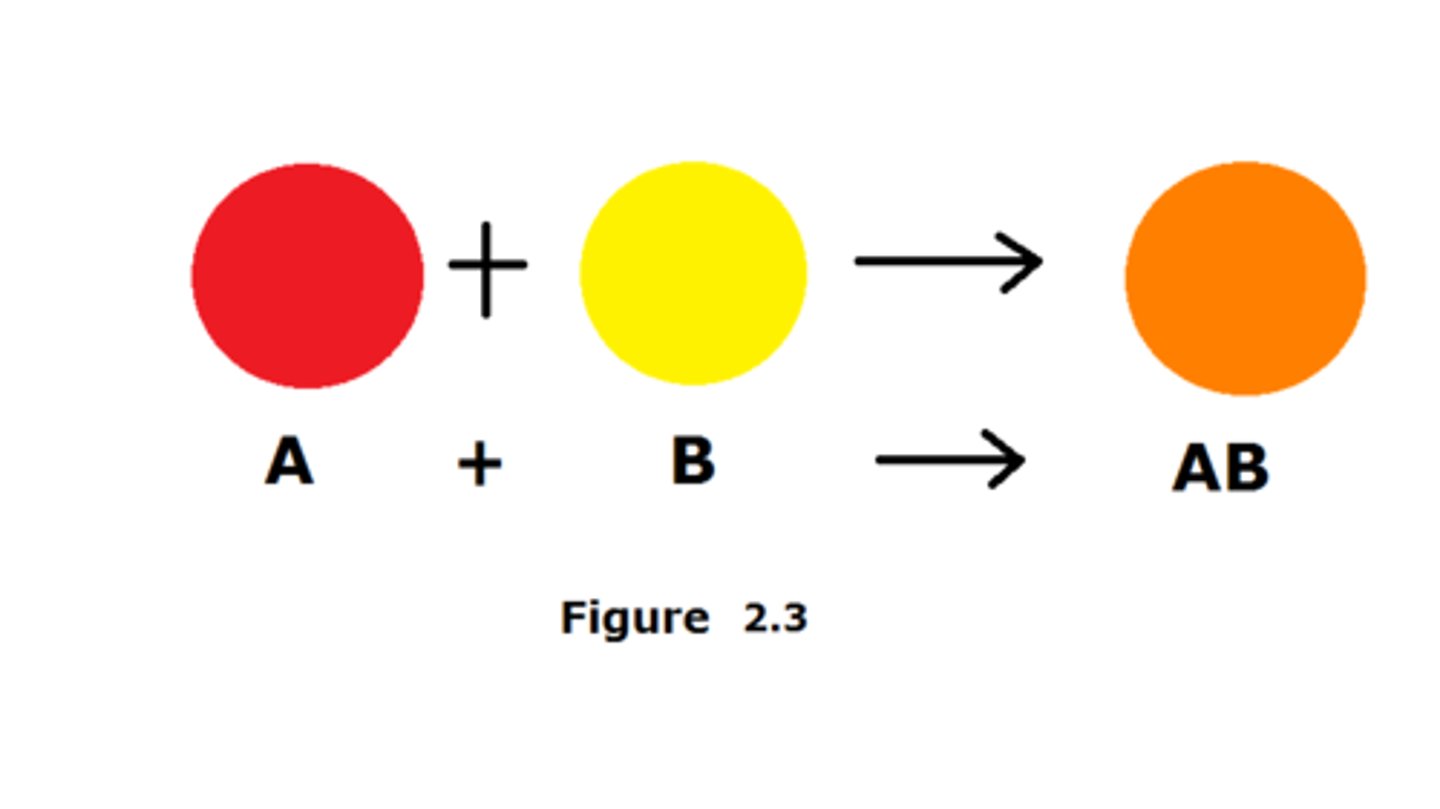
synthesis reaction
a reaction in which two or more substances combine to form a new compound
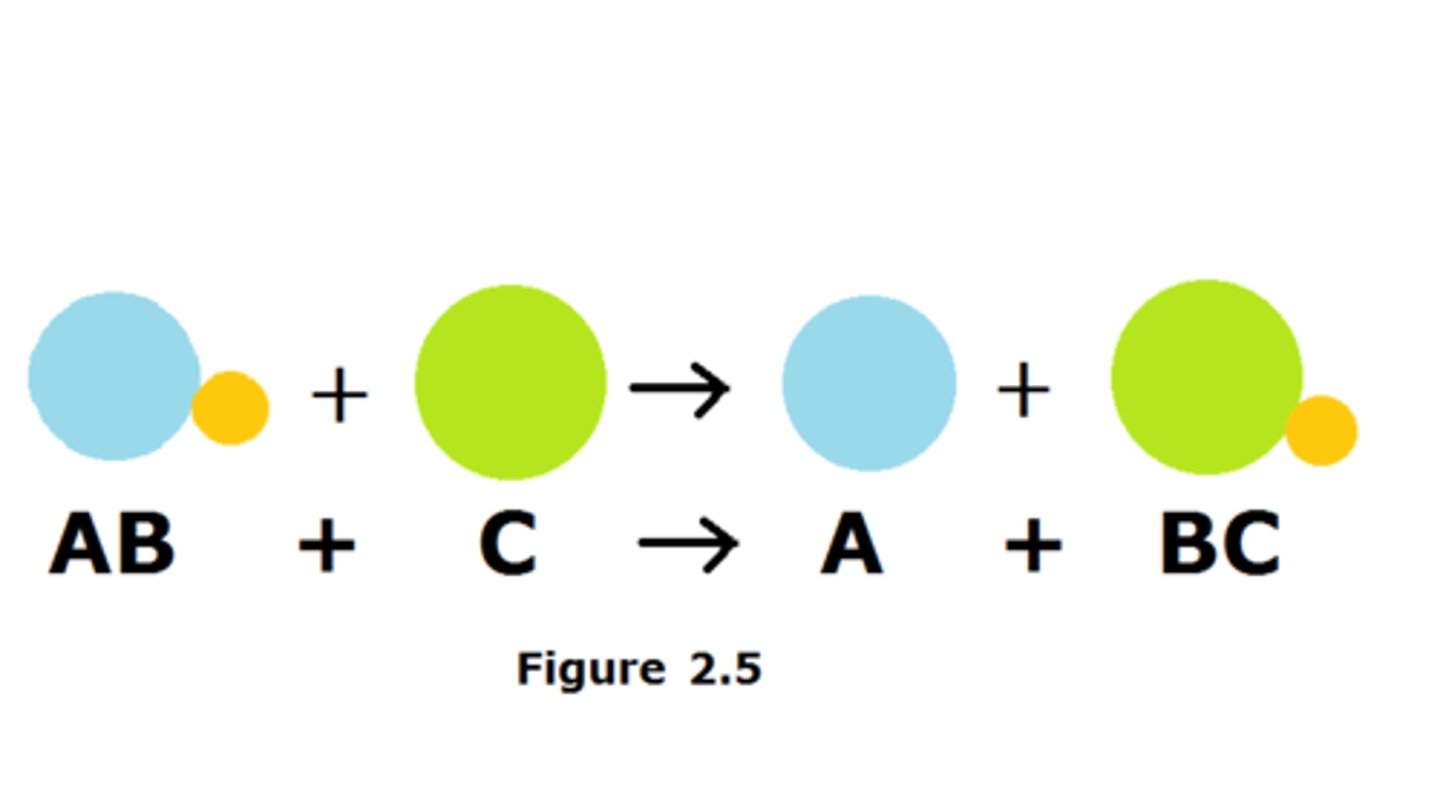
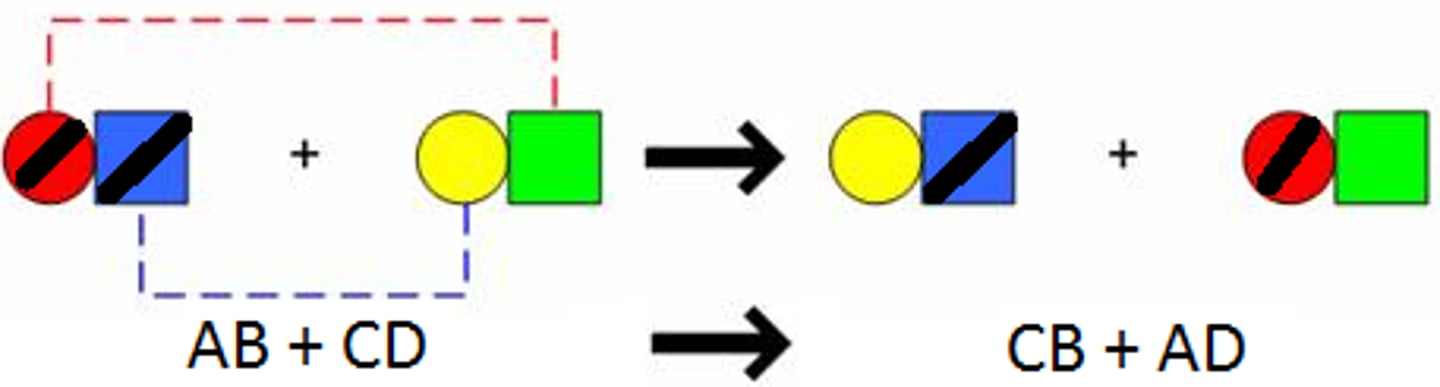
single replacement reaction
a chemical change in which one element replaces a second element in a compound
double replacement reaction
a chemical change that involves an exchange of positive ions between two compounds
combustion reaction
a chemical reaction that occurs when a substance reacts with oxygen, releasing energy in the form of heat and light
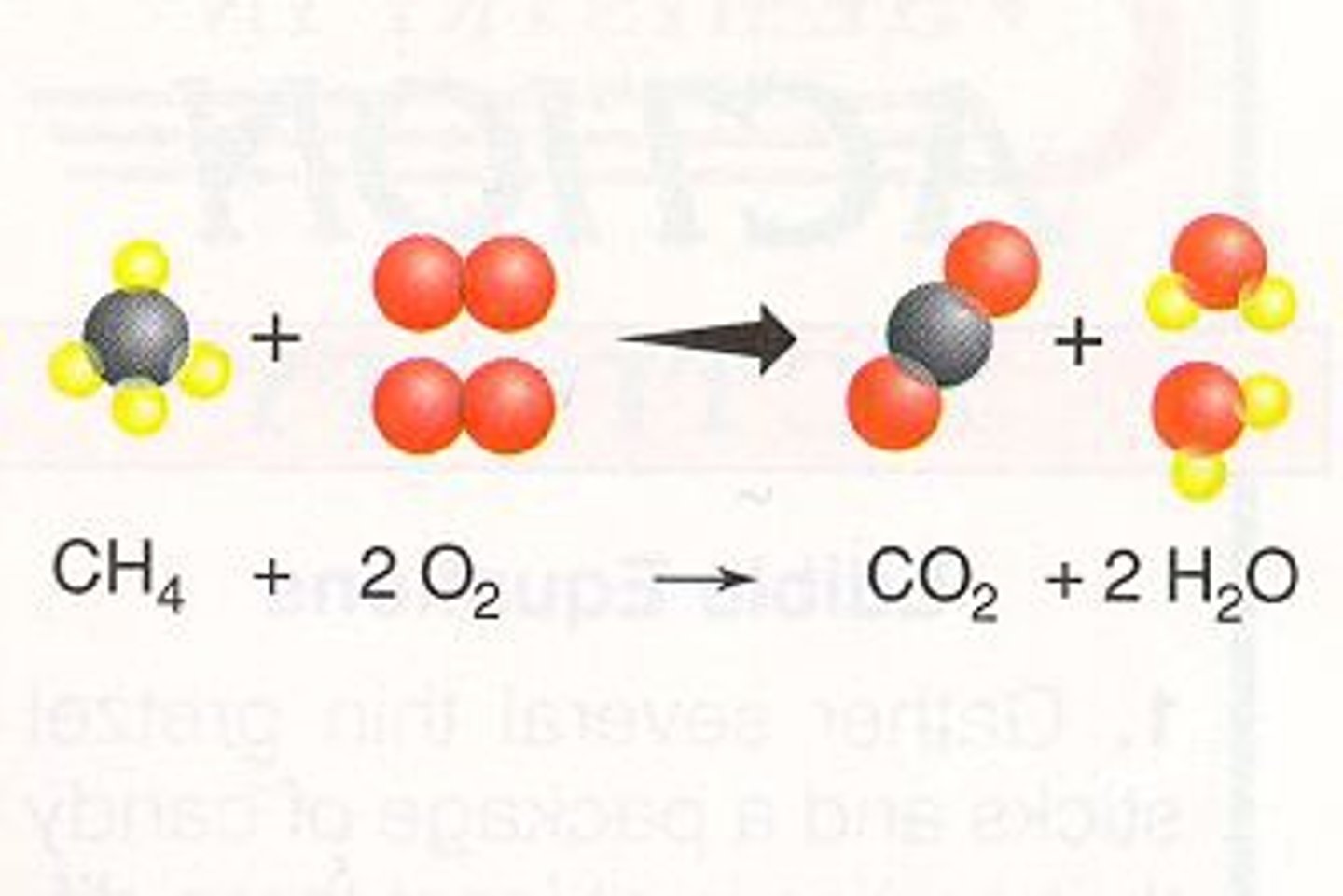
precipitation reaction
a reaction in which an insoluble substance forms and separates from the solution

soluble
capable of being dissolved

Insoluble
incapable of being dissolved

precipitate
A solid that forms from a solution during a chemical reaction.

reaction rate
the rate at which reactants change into products over time
Temperature & reaction rate
usually the higher the temperature the faster the reaction rate
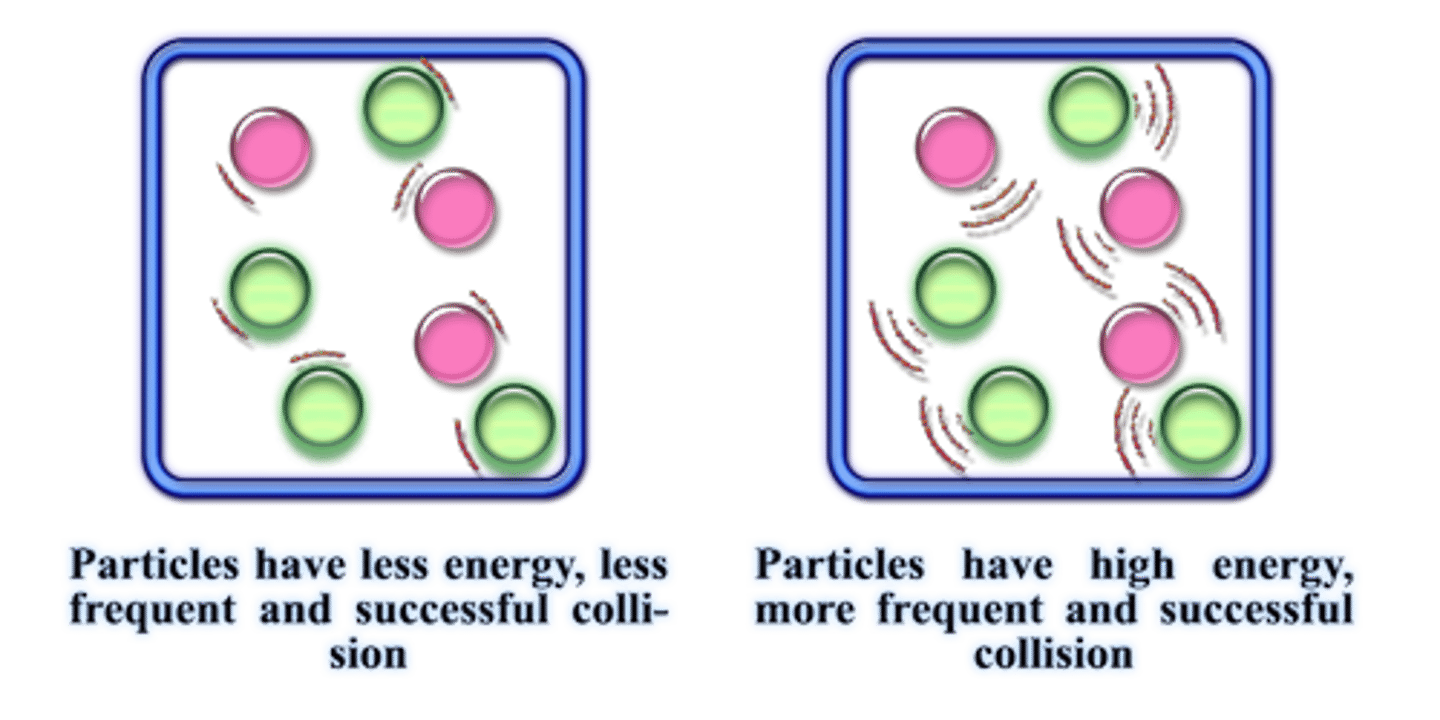
Concentration & Reaction Rate
the higher the concentration of starting materials, the more rapidly the reaction takes place (more collisions taking place). as more reactants are consumed the rate of reaction slows down
Agitation
Stirring up the reactants - increasing the reaction rate

catalyst
substance that speeds up the rate of a chemical reaction
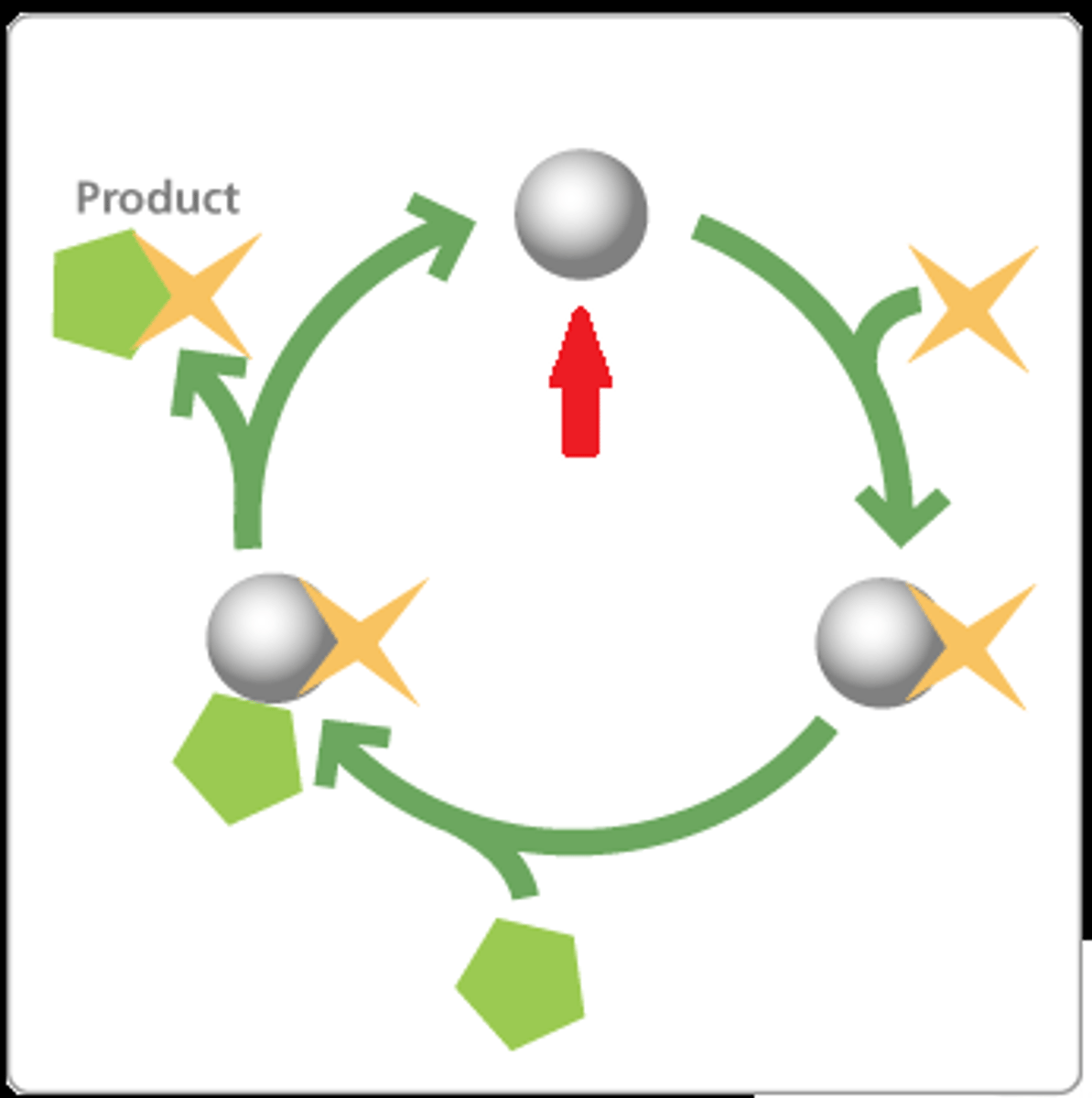
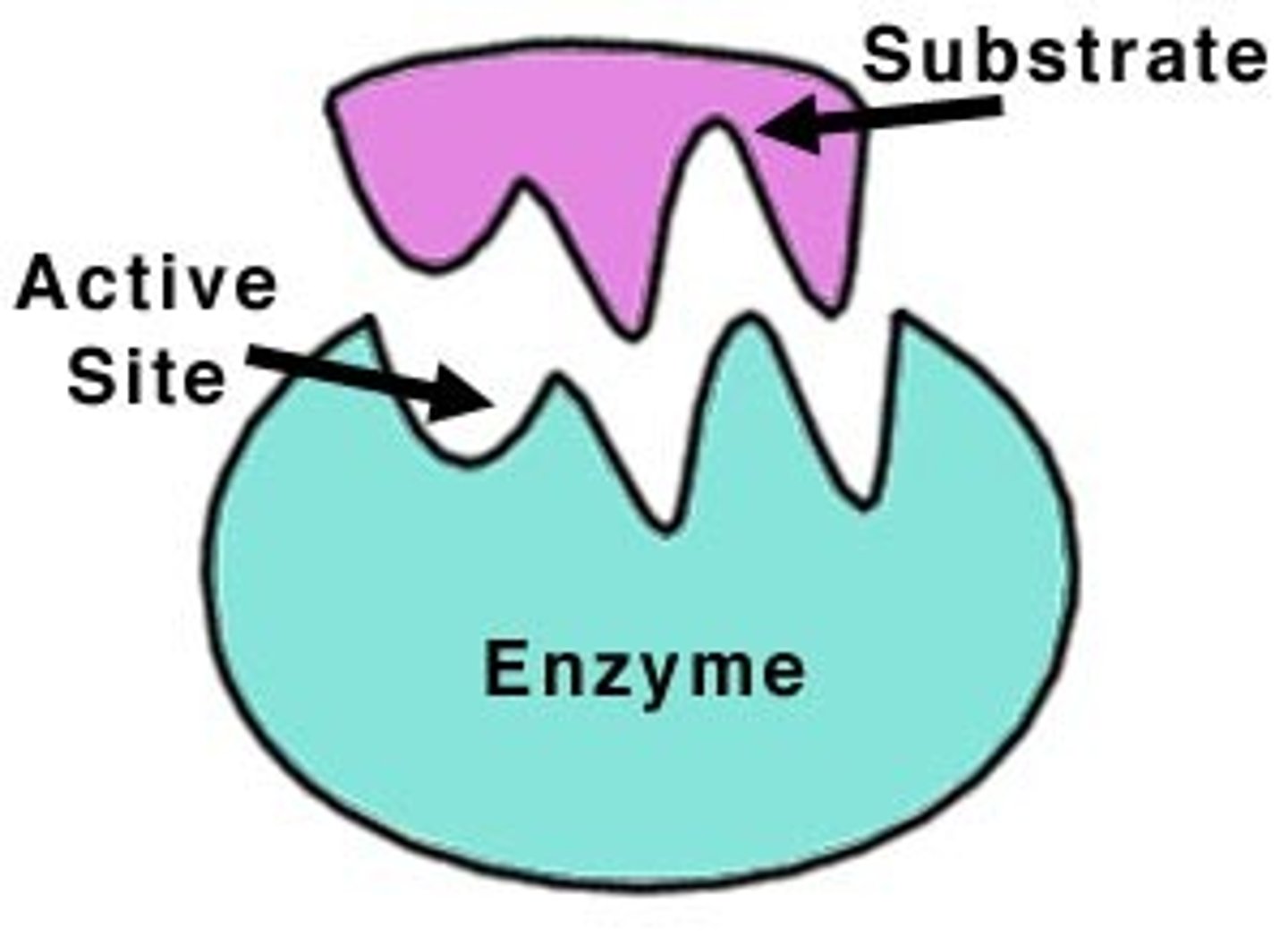
Enzymes
Catalysts for chemical reactions in living things