midterm study guide (units #1-3)
UNIT 1 ✰
^^si units/metric units:^^
measuring length: the measurement or extent of something from end to end
volume: the amount of space an object takes up
- the base unit of volume in the metric system in the liter is represented by L or l
standard: 1 liter is equal to one cubic decimeter
metric units
1 liter (L) = 1,000 milliliters (mL)
1 milliliter (mL) = 1 cm^3 (or cc) = 1 gram*
*when referred to water
- graduated cylinders are going to be used in order to find the ^^volume of liquids and other objects
- measure the ^^volume of a regular object^^ using the formula length x width x height
mass: amount of matter in an object
- the base unit of mass in the metric system in the gram (g)
^^significant figures:^^
the atlantic-pacific rule: determining the # of significant digits
if a decimal point is ^^present^^, start at the ^^pacific side (left) of the #^^, look for the first nonzero digit, that and everything after is significant
ex.: 0.00238930 cm = 6 significant figures
if a decimal point is ^^absent^^, start from the ^^atlantic side (right) of the #^^ and look for the first nonzero digit, that and everything before it would be significant
ex.: 128,021,600 = 7 significant figures
significant digits in calculations:
^^multiplication/division^^ - your calculated value cannot be more precise than the least precise quantity used in your calculation
RULE: keep least # sig figs in answer
ex.:
3.0m x 125.8m x 710m = 267,954m^3
(2 s.f., 4 s.f., 2 s.f.) → the answer should be rounded to 2 sig figs
^^addition/subtraction^^ - your calculated value cannot be more precise than the least precise place value of the measurement used in your calculation
RULE: keep least # of decimal places in answer
ex.:
12,003cm + 56.2cm = 12,059 cm
(0 decimals, 1 decimal) → keep your answer with zero decimals
^^base units vs. derived units:^^
^^base units^^ - units that are defined through a measurement process
ex.: meter, second, kilogram, kelvin, mole
^^derived units^^ - units that can be expressed as combinations of base units
ex.: area, volume, speed, acceleration, density, energy
^^mass vs. weight:^^
^^mass^^ - how much MATTER something has
^^weight^^ - depends on gravity (force pulling on object toward earth, moon, or other planet)
^^density:^^ amount of mass in a given space; ratio of mass to volume
formula on table t: d=m/v
^^percent error:^^ measurement of the % that the measured value is “off” from the accepted value
table t formula: % error = ((measured value - accepted value)/accepted value)x100
- if ^^negative^^, your measured value was ^^less^^ than the accepted value
- if ^^positive^^, your measured value was ^^greater^^ than the accepted value
UNIT 2 ✰
^^matter/pure substances:^^
matter:
- matter exists in ^^three main states^^: solid, liquid and gas
- matter can be divided into ^^two categories^^: substances and mixtures
- matter is considered a ^^pure substance^^ if its composition is the same throughout the sample
substances/compounds:
- substances are ^^homogeneous^^—they have the same composition and properties throughout
ex.: water, salt, vinegar
2 types of substances: elements & compounds
elements: ^^CANNOT^^ be decomposed with chemical methods
ex.: C, H2, Ca, Mg, Na, O2
(also only contains 1 element symbol, count the capital letters)
compounds: ^^CAN^^ be decomposed with chemical methods
ex.: H20, NH3, H2SO4, CH4
(contains multiple element symbols)
- most elements are metals
- compounds are always chemically combined in definite proportions by mass
- the law of definite proportions states that types of atoms in a compound exist in a fixed ratio
diatomic molecules: these elements, as ^^pure substances^^, are always found bound to themselves
these include: H2, N2, O2, F2, Cl2, Br, I2
^^compounds vs. mixtures:^^
mixtures: two or more substances mixed
(this means they are not bonded to each other, are not found in fixed proportions, and cannot be written in a chemical formula)
types of mixtures:
| <<homogeneous<< | <<heterogenous<< |
|---|---|
| solutions | 2 different substances |
| salt dissolved evenly throughout water | salt & sugar |
| at least two pure substances but only one is visible- aqueous: NaCl (aq) | x |
| alloy: mixture of metals- steel: carbon & iron- brass: copper & zinc | x |
comparing compounds & mixtures:
| <<compound (chemically combined)<< | <<mixture (physically combines)<< |
|---|---|
| water (H2O) | cereal and milk |
| the H (hydrogen) and O (oxygen) are chemically bonded | 2 or more substances |
| - different chemical properties than the elements that make it up- H2O has different properties than H2, a colorless gas and O2, also a colorless gas | - each substance maintains its own chemical properties in a mixture. |
| - can be written as a chemical formula | - cannot be written as a chemical formula |
| - elements in definite proportions- 2 H: 1 O.- this ratio is always the same for this compound | - different proportions- you can have the cereal in the same proportion as the milk (1:1), you can put twice as much cereal (2:1), you can put one drop of milk (1:???) |
^^particle diagrams/separation of mixtures:^^
particle diagrams: charts that show the phase of matter and the type of matter of a substance or a mixture
- circles are usually used to represent an atom
- ^^different colors/shades^^ represent different elements
- space is not to scale, but is still ^^approximate^^ (if two particles look closer together than others, they are)
separation of mixtures:
- ^^distillation^^: separation based on of different liquids boiling
- ^^filtration^^: separation based on particle size (separates heterogenous mixtures)
- ^^chromatography^^: separation based on polarity & solubility (the more soluble a molecule is, the higher it will travel up the paper)
^^polarity^^: how much electric charge is separated in a molecule
^^solubility^^: how easily a substance dissolves
^^properties of matter/physical vs. chemical changes:^^
| <<physical<< | <<chemical<< |
|---|---|
| same substance, different state | something new is formed |
| H2O(s) + heat → H2O(l) | 2H2(g) + O2(g) → 2H2)(g) |
| phase changes | making new compounds |
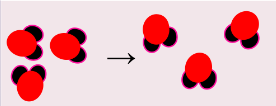 | 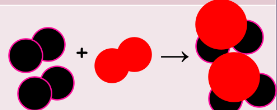 |
<<key words for changes<<<<:<<chemical: rust, bubble, fizz, burning, color change, reacts with
physical: melt, boil/vaporize, freeze, condense, dissolve, soluble, sublime, deposit
temperature & energy:
^^temperature scales:^^
- ^^celsius (°C)^^ - based on boiling point/freezing point of water
- ^^kelvin (K)^^ - based on absolute zero
- ^^fahrenheit (°F)^^ - used in U.S.
table t: K = °C + 273
energy: ability to do work or produce heat
^^potential energy (PE)^^: stored energy
- held in chemical bonds (attachments) between atoms
- how far apart particles are
^^kinetic energy (KE)^^: energy of motion
- proportional to temperature
^^heat^^: flow of energy due to temperature difference
- always flows from high temperature to low temperature
endothermic vs. exothermic:
^^endothermic^^ - energy is absorbed (energy enters)
exothermic - energy is released (energy exits)
an exothermic process is one that produces heat (exo = outside, thermic = heat)
an endothermic process is one that absorbs heat (endo = inside, thermic = heat)
phase changes:
^^solids^^- matter is held in a rigid form—the substance has a definite volume and shape
- solids maintain their shape no matter what container they are placed in
^^liquids^^- liquid particles can move past each other—the substance has a definite volume, but no definite shape
- liquids fill a container up to their volume
^^gases^^ - gas particles are spread out and do not attract each other—the substance has no definite volume and no definite shape
- gases expand to fill up the entire container they are placed in
energy is required both to increase temperature and to change phases
^^heating/cooling curves^^:
graphs of temperature vs. time
- show the phase changes of a substance- as temperature stays ^^constant^^ (no slope/plateau) represents a phase change
- places with ^^slopes^^ (diagonal) indicate a temperature change
- endothermic (heating curve) or exothermic (cooling curve)
^^heat calculations:^^
heat energy formula (change in temperature):
q = MC∆T
q = heat (in joules)
m = mass of substance (in grams)
C = specific heat capacity in a substance (in J/g * °C)
∆T = change in temperature (in celsius or kelvin)
heat of fusion/vaporization:
the heat energy formula for change in temperature ^^does not apply^^ for phase changes
^^heat of fusion^^: the amount of heat required to melt a substance
^^heat of vaporization^^: the amount of heat required to boil a substance
these are measured in J/g!
UNIT 3 ✰
^^atomic theory:^^
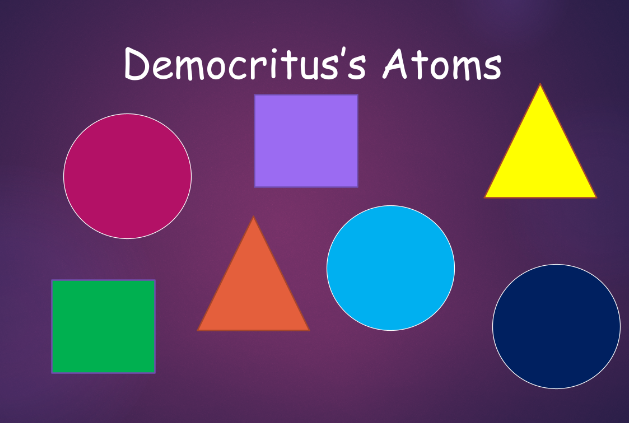
democritus (atomism):
- everything is composed of atoms, (from “atomos,” greek for indivisible)
- between atoms are empty space
- atoms are indestructible
- atoms are always in motion
- there are infinitely many atoms and infinitely many types of atoms, which differ in shape and size
john dalton (1766-1844):
atomic theory becomes widely accepted, he proposed the following:
elements are made of extremely small particles called atoms
for one type of element, atoms have identical properties (size, mass, etc.), atoms of different elements have different properties
atoms cannot be subdivided, created, or destroyed
atoms of different elements combine in simple, whole-number ratios to form chemical compounds
in chemical reactions, atoms are combined, separated, or rearranged (NOT TRANSFORMED)
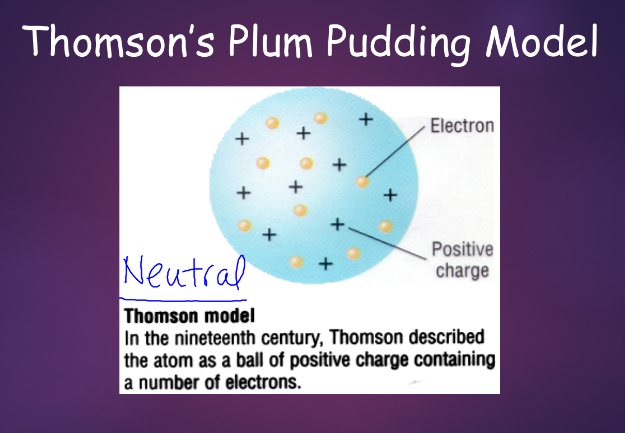
j. j. thomson (1856-1940):
using a device called ^^acathode ray tube^^, thomson discovered the ^^electron^^ in 1897
- thomson measured the rays inside and found that they had mass, but were much lighter than ^^hydrogen^^, which was known to be the lightest element at the time
- he also measured that they had a negative charge and it was quickly recognized that electrons carry negative charge in atoms
ernest rutherford (1871-1937):
in what is now known as the gold foil experiment, rutherford discovered the atomic nucleus
- with the plum pudding model, it was expected that positively charged particles would pass through or barely bounce off atoms
- things did not happen that way
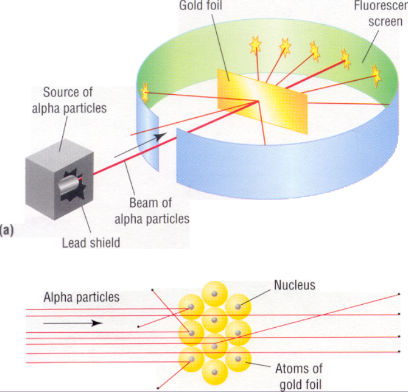
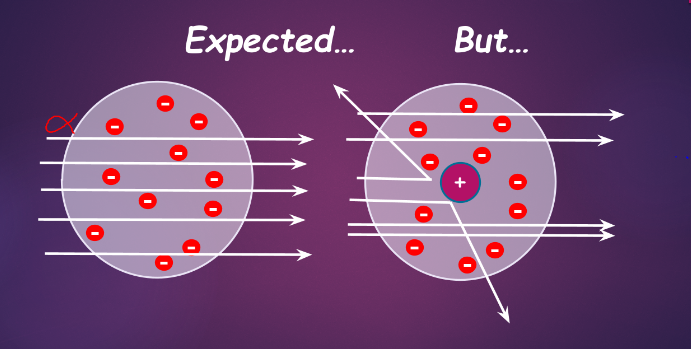
| ^^observation^^ | <<conclusion<< |
|---|---|
| most alpha particles pass through gold foil | atom is mostly empty space |
| some alpha particles were deflected | atom has a small, dense, positive core (nucleus) |
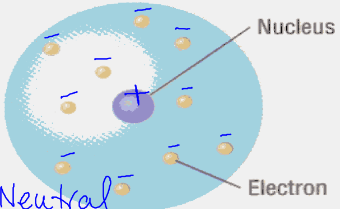
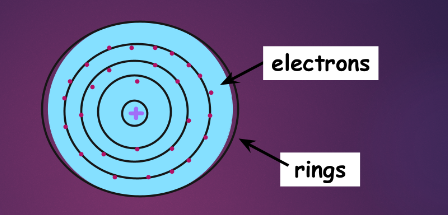
bohr’s planetary model:
modern theories:
^^discovery of isotopes^^ - not all atoms of the same element have the same mass
^^discovery of neutrons^^ - components of the nucleus figured out
^^discovery of particle-wave duality^^ - electron location are based on probability
^^development of quantum physics^^ - electrons can only have certain amounts of energy
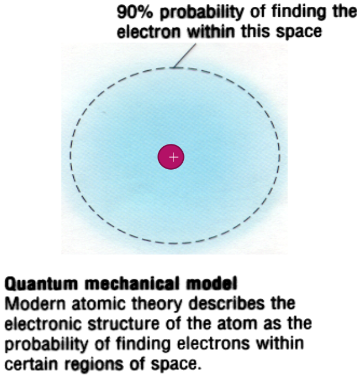
the wave-mechanical model:
- the nucleus is still where rutherford concluded it should be, but locations of electrons get complicated
- electrons are now found in orbitals—regions in which an electron with a particular amount of energy is most likely to be located
^^subatomic particles:^^
- an amu, or atomic mass unit is defined as 1/12th the mass of a carbon-12 atom, or approximately 1.66054 * 10^-27 kg
- protons and neutrons are classified as nucleons, or particles found in the nuclei of atoms
![]()
how to read atomic notation:
![]()
chemical symbol - indicates what type of element
atomic number - indicates how many protons are in the nucleus of any atom of the element
mass number - indicates the total number of nucleons in the sample/atom of the element we are talking about
features of atomic number and atomic mass:
- every element has its own atomic number (every element is defined by its atomic number)
- the charge of the nucleus is equal to its atomic number (+1 * atomic number)
- atomic mass (similar to mass number) is roughly equal to the sum of protons and neutrons in the nucleus
- aside from atomic notation, atomic mass can be written in word form as element-mass number (ex.: nitrogen-14)
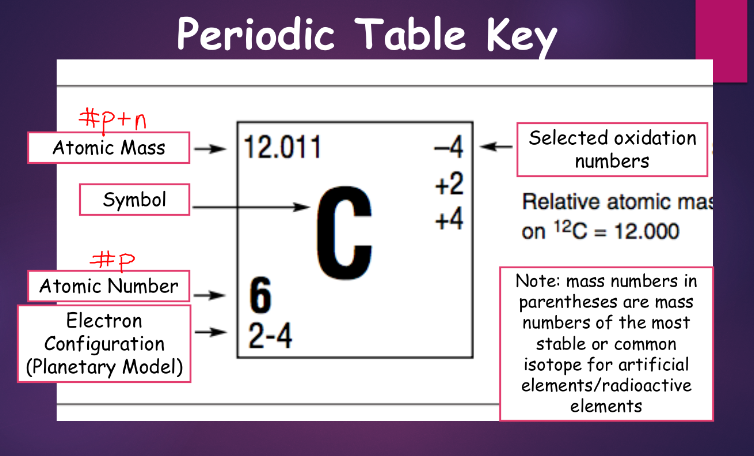
why is the mass on the periodic table not a whole number?
atoms cannot have fractions of protons or neutrons; on the periodic table, atomic mass is shown as a weighted average of all of an element’s isotopes
important calculation methods:
^^number of protons^^ - look up the atomic number of element
^^number of electrons in a neutral atom^^ - equal to the number of protons/atomic number
^^number of neutrons^^ - mass number minus atomic number
^^weighted averages:^^
weighted average = (weight fraction(a) * value (a) + weight fraction(b) * value(b)+…)
atomic mass is determined by using weighted averages; this relies on the relative abundance of each isotope, which is a measure of what part of a sample of any element consists of elements of that specific isotope
recall: bromine-79 has 51% relative abundance, bromine-81 has 49% relative abundance
calculating a weighted average for atomic mass, do the following:
- convert relative abundance percentages to fraction or decimal values
- multiply each fraction or decimal by the corresponding atomic mass
- add up all the products
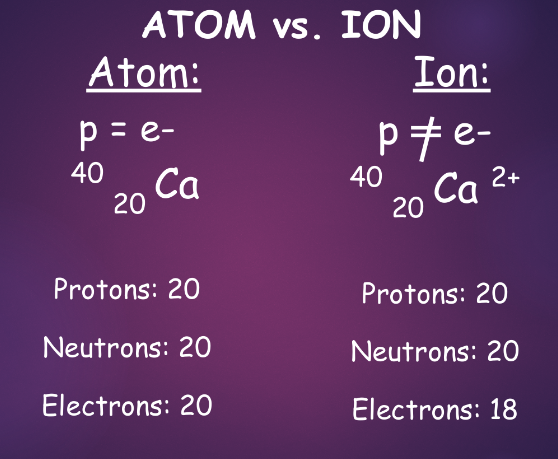
^^ions/charged atoms:^^
ions form by changing the number of electrons
- lose electrons → form a cation (+)
- gain electrons → form an anion (-)
- think of these in terms of adding and subtracting negative numbers
showing ions in atomic notation
- the charge of an ion is written in the top right, indicating number and type of charge
- by convention, we do not need to write charges of 1—just show what type
^^electrons in the bohr model:^^
bohr (planetary) model:
recall: electrons travel around the nucleus in shells
these shells are also known as principal energy levels/PELs
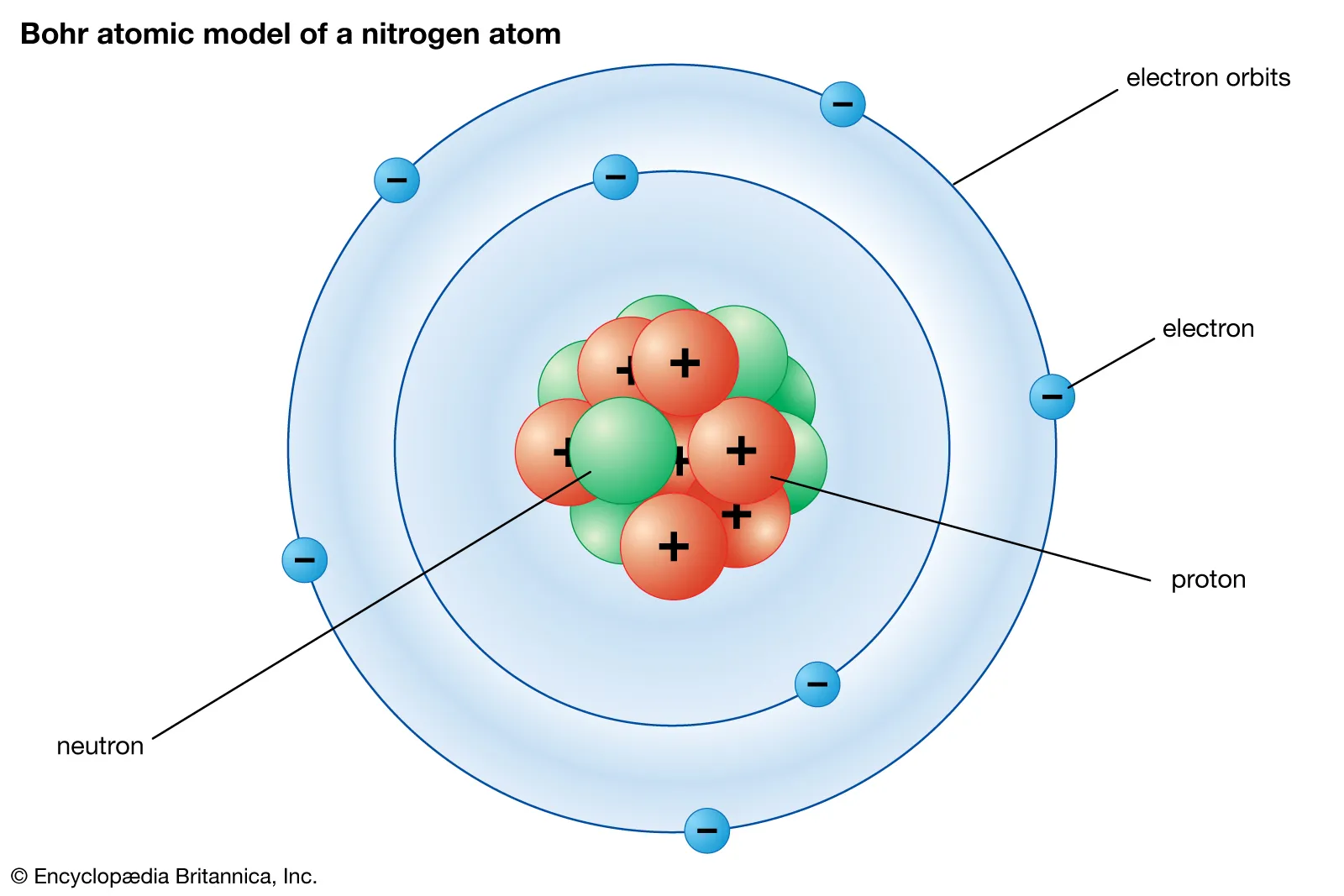
PELs:
- fixed distances from the nucleus
- can hold a specific number of electrons
- has a definite amount of energy- scale in energy based on distance from nucleus (more distance = more energy)
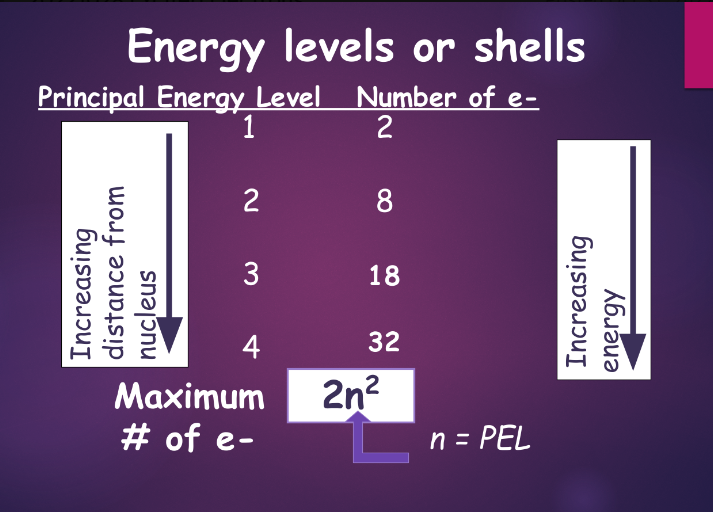
how to fill electron shells:
- in an atom in its normal state, electrons occupy the lowest PEL first
- electrons cannot exceed the maximum number for each PEL as per the 2n^2 rule—the maximum number of electrons in any PEL is 2n^2, where n = PEL number
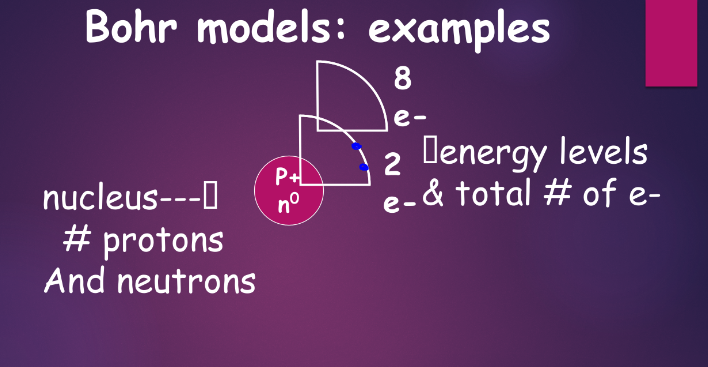
^^electrons in the wave-mechanical model:^^
wave-mechanical model revisited:
- in contrast to the planetary model, most probable location of electrons are used
- electrons are in orbitals
- there are different sublevels of orbitals, labeled as s, p, d, and f
- compared to the bohr model, as we increase the PEL number, we add a letter
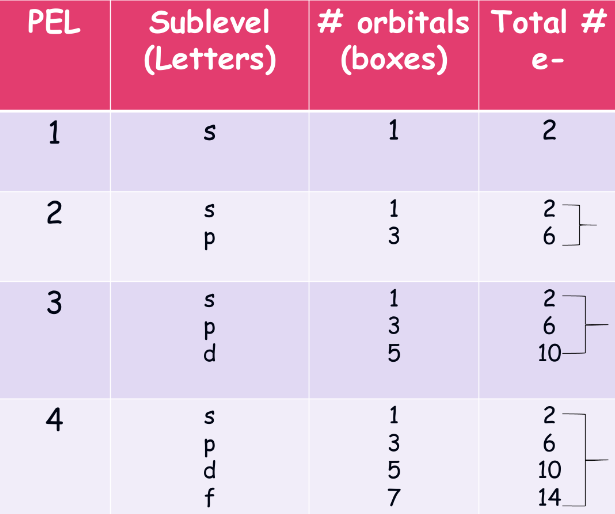
orbital diagrams:
this is a more specific way to illustrate and characterize electron energy levels
the simple way to think of orbital diagrams and how to build them—imagine you are managing a hotel and assigning guests to rooms.
- electrons are your guests
- PELs are floors (1, 2, 3, 4, etc.)
- sublevels are hallways, with s being closest to the stairs and f being the furthest (s, p, d, f)
- orbitals are rooms, but assume all the rooms in a hallway are the same in terms of convenience (s has 1 room, p has 3 rooms, d has 5 rooms, and so on.)
some rules for managing the hotel:
the hotel only serves parties of 1, every person that comes in is by themselves
give the guests the most convenient rooms first, this takes priority above everything else
two guests may share a room if they need to, but no more than that
two guests may not share a room if there is another empty room in the same hallway
the hotel rules, but in chemistry terms:
the hotel only serves parties of 1, every person that comes in is by themselves
*give the guests the most convenient rooms first, this takes priority above everything else
**two guests may share a room if they need to, but no more than that
***two guests may not share a room if there is another empty room in the same hallway
^^*aufbau principle^^: electrons fill in order of increasing sublevels
^^**pauli exclusion principle^^: there is a maximum of 2 electrons per orbital (we represent them using arrows—one up, one down)
^^***hund’s rule^^: within a sublevel, fill in each orbital with an electron before you pair them
^^excited electrons:^^
electron configurations recap:
wave-mechanical model: 1s^2 2s^2 (this is more specific)
bohr model: 2-8-18 (this is more general)
- these are for atoms in the ground state—when all electrons in the atom occupy the lowest available orbitals or energy levels
- this contrasts with what we will be talking about today, excited states—when one or more electrons absorb energy and no longer occupy the lowest available orbitals or energy levels
changing electron energies:
because we know that electrons have certain amounts of energy, it makes sense to imagine that if we could add energy to an electron, it could change energy levels; this is the case
- electrons can absorb energy to jump to a higher energy sublevel or a higher PEL
ex.: an Mg atom, with electron configuration of 2-8-2—adding energy might cause an electron to jump from PEL 2 to PEL 3, giving us an excited state electron configuration of 2-7-3
- this can happen with multiple electrons at the same time—ex.: 2-6-4, 2-5-5—and with electrons from different PELs—ex.:1-5-6
- with orbitals, it can happen between sublevels—1s^2 2s^2 2p^5 3s^2 3p^1
some rules still apply:
- 2n^2 rule still holds
- pauli exclusion principle still holds
connecting this to the dangerous stuff:
when an electron jumps to a higher energy level, it absorbs energy
- this process is called absorption
- THIS IS NOT PERMANENT!
the additional energy is eventually released as the atom returns to the ground state
- the excess energy is emitted as light
- this process is called emission
in the demonstration, both these things happened
- what is the source of energy for absorption?
- the flame/the heat
- what is the sign that emission is happening?
- the different colors that can be seen
- why are there different colors?
^^atomic spectra:^^
different colored flames:
the energy that is released from emission has a characteristic wavelength (and color)
- every transition for every element produces a specific wavelength (or specific “color”); when you observe an element in a flame, you see all of them together
- using a prism, this light can be separated into its individual “colors”
- the result is a unique bright line spectrum that can be used to identify the presence of that element
light and atomic spectra (bright line spectra):
^^spectroscopy^^ - field of study that measures electromagnetic spectra created by matter interacting with electromagnetic energy
- three things are needed to do spectroscopy: a light source, an interaction, and a measuring device
- instruments called spectroscopes, separate and show the wavelengths of light present
- atomic emission spectra produce narrow lines of color called bright line spectra
- each line corresponds to an exact wavelength
- every element has a unique set of spectral line, the elements fingerprint
^^flame test^^ - demonstrates the emission spectrum of a substance
- completed by heating elements to high temperatures so they may enter excited state
- characteristic color will be emitted as excited electrons return to ground state
- used to determine metal ion presence in unknown substance
uses for spectroscopy:
- ^^emission spectroscopy^^ - figure out the identity of an unknown element
- ^^absorption spectroscopy^^ - detect an element among many others (like on another planet)
- ^^NMR and raman spectroscopy^^ - make a new molecule, and you can figure out the structure of what you’ve made
- ^^practical applications^^ - space exploration, blood analysis, chemical analysis, environmental monitoring, etc.