Chapter 14 translation
1/32
Earn XP
Description and Tags
genetics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
Polypeptide
Long chain of amino acids linked by peptide bonds
Peptide bonds
Covalent bond that forms been the carboxyl end of an amino acid and the amino acid of the next bond
Calmodulin
Present in all eukaryotes
A small protein that is a single polypeptide chain with an overall tertiary structure
Has alpha helices (secondary structure)
Hemoglobin
The protein inside red blood cells that carries oxygen in the bloodstream
Has 4 polypeptides (2 a-globin’s and b-globin’s) making it a quaternary structure
Structural organization of Hemoglobin
Primary structure
Polypeptide: a sequence of amino acids
Secondary structure
Alpha helix: Partial folding
Tertiary structure
Globin: Complete folded polypeptide
Quaternary structure
Hemoglobin: 4 globin’s
Codon
A sequence of 3 nucleotides of DNA that code for an amino acid
mRNA codons- 5’ to 3’ direction
Starting codon
AUG (methionine)
Ending codon
UAA, UAG, UGA (these DON’T code for an amino acid)
Reading frame
A linear sequence of codons in a nucleic acid defined by a start codon and a stop codon
Characteristics of the genetic code
Unambiguous
Each of the 61 triplets code for only one of the 20 amino acids
Degenerate
Most amino acids are encoded by more than one codon
Universal
MOST living organisms use the same code but there are exceptions
Commaless
No breaks between the codons in a reading frame (read continuously as groups of 3)
Non- overlapping
Triplets in a reading frame are in a tandem sequence and don’t overlap
Central Dogma (The flow of genetic code)
DNA → RNA → Protein
DNA is the long-term storage of genetic information.
RNA is the temporary copy used to build proteins.
Proteins do the work in the cell and determine traits.
The flow of genetic code (Central dogma)
DNA (Template/ antisense strand)
The 3’ to 5’ DNA template strand is used to make mRNA
Transcription
RNA polymerase reads the DNA template strand and builds mRNA in the 5’ to 3’ direction
Translation
Ribosomes read the mRNA codons (in groups of three) and converts them into a chain of amino acids
Protein (polypeptide)
Amino acids link to form a protein which preforms functions in the cell
Spontaneous mutations
DNA inside the cell can be exposed to radiations and molecules that react with it and cause chemical alterations (some of which can be permanent)
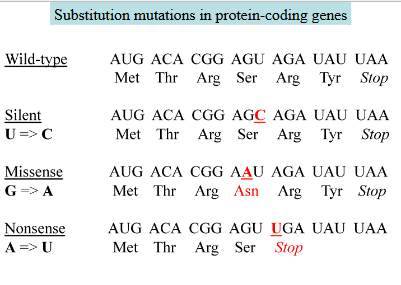
Substitution mutations
A base replaced by a different base in DNA causes a permanent single codon change.
EX.
Silent: The resulting new codon codes for the same amino acid as the original one
Missense: The new codon codes for a different amino acid
Nonsense: the new codon is a stop codon, resulting in a shorter polypeptide
Note:
Mutations can be cause by errors in DNA replication and can be copied into mRNA’s
Insertion of a single base results in frameshift mutation
Insertions are due to errors in DNA replication
Frameshift mutation occurs when one base (one letter in a code) of a codon is inserted or deleted
Trinucleotide repeat expansion
Starts with 8 CAG gene
Two strands separate
Then replicate
Hairpin forms on newly synthesized gene
causes part of the template strand to be replicated twice and increases the number of repeats on the newly synthesized strand
two strands of the DNA molecule separate
the strand with extra CAG serves as a template for replication
resulting DNA molecules have 5 extra CAG strand repeats
Huntington’s disease Trinucleotide repeat expansion
A neurodegenerative disorder (autosomal dominant) caused by abnormal repeats of CAG gene (codes for glutamine in mRNA in the Huntington gene) resulting in polyglutamine Huntington gene
Basic requirements of translation
mRNA
charges transfer RNA’s (tRNA)
ribosome
no primers
other: initiation factors, elongation factors, energy sources
Transfer RNA’s
An amino acid is attached to the 3’ end of the tRNA. The Anti-codon loop base pairs with a codon in the mRNA
Unusual bases found in tRNA’s
Post transcriptional modified of bases in tRNA’s result in unusual bases
Inosine: a modified adenine
Inosine
a post transcriptionally modified adenine that can occur at the wobble site of tRNA’s
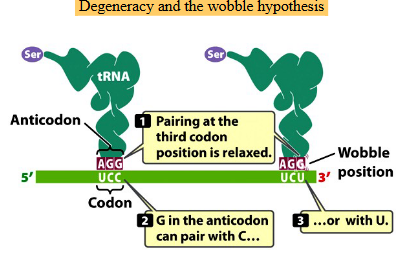
The wobble hypothesis
The interaction between the 3rd position of the codon in the mRNA and the 1st position of the anticodon in the tRNA
Some tRNA bases can pair with multiple mRNA bases at the wobble position, allowing translation to occur without the cell needing to synthesize all 61 tRNA’s
Basically explains how one tRNA can read multiple codons as it doesn’t need a perfect base pairing
why one tRNA can read multiple codons for the same amino acid?
Pairing at the third codon position is relaxed
The first two bases pair normally, but the third can “wobble.”G in the anticodon can pair with C or U
That’s why one tRNA can read multiple codons for the same amino acid.
Charging of tRNA’s (attaching an amino acid to it’s tRNA)
The carboxyl group of the amino acid is covalently attached to the 3’ end of a tRNA
Charging is catalyzed by 20 different aminoacyl tRNA synthases
when the correct amino acid is added
the enzyme recognizes the amino acid by it’s R group
the enzyme recognizes the tRNA by it’s shape and base sequence
Energy is spent during this process in order to create a new peptide bond in the ribosome
Recognition of tRNA’s by aminoacyl tRNA syntheses
positions in…
Blue- Can’t be used to differentiate among tRNA’s (the same in all tRNA’s)
Red- Important for recognition of tRNA’s by one synthetase (unique in tRNA’s)
Yellow- Are used by more than one synthetase (shared in some tRNA’s)
Steps for charging tRNA when the correct amino acid is added
Step one: Amino acid activation
The amino acid (AA) is transformed into aminoacyl adenylic acid (AA-AMP). This is the energy consuming step that requires ATP in order to create a new peptide bond
Step two: Charging
The AA-AMP loses its AMP and the carboxyl group of the amino acid attaches to the 3’ end of a tRNA
This results in a aminoacyl tRNA (a charged tRNA)
The prokaryotic ribosome
Large particles of rRNA and proteins where translation occurs
Consists of 2 svedberg subunits:
Small subunit: 30S
Large subunit: 50S
Complete ribosome = 70S
Svedberg units: The rate at which particles sediment in a centrifugal field (depends on weight, shape, and size) and can be used for measuring large molecules or large cell components such as ribosomes and organelles.
Sites found in all ribosomes
Peptydyl (P)
Aminoacyl (A)
Exit (E)
Steps in Prokaryotic translation initiation
Where AUG is on the P site IF-3 bonds to the small subunit then mRNA bonds to the IF-3 small subunit
f-Met-tRNA (a complex forms with IF-2 and GTP) (found in prokaryotes) binds to AUG codon
The large ribosomal subunit joins the complex
The process requires GTP as an energy source + a series of initiation factors (IF proteins)
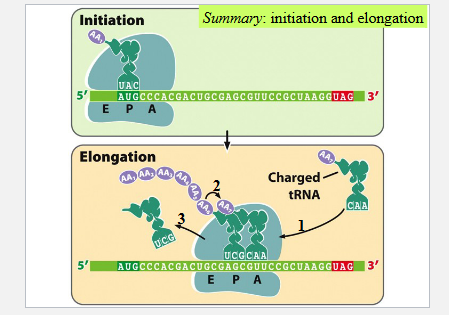
Elongation of the polypeptide chain
EF-Tu (an elongation factor) and GTP facilitate binding the second tRNA to the second codon at the A site
The amino acid on the first tRNA is transferred and forms a peptide bond with the amino acid on the second tRNA (a dipeptide forms)
The first tRNA moves to the E site
The mRNA is shifted to place the second codon in the P site and to bring the third codon into the A site
The tRNA carrying the third amino acid binds the third codon on the A site
The dipeptide on the second tRNA is transferred to form a tripeptide attached to the third tRNA
The second tRNA moves to the E site with the help of EF-G and GTP
The third codon moves to the P site
translation continues...
Initiation and elongation summary
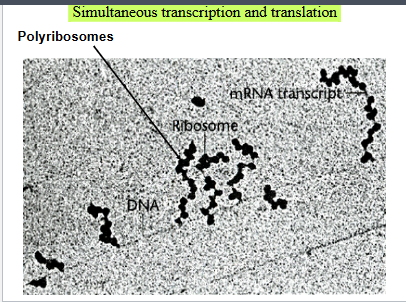
Simultaneous transcription and translation in prokaryotes
There is no nucleus so multiple ribosomes can translate the same mRNA at once creating a polyribosome
Termination of translation
A STOP codon moves to the A site of the ribosome
RF1 (release factor 1) binds to stop codon
The GTP dependent RF3 releases the polypeptide chain from the last tRNA and the entire translation complex dissociates