9/30 Immune Diversity & Tolerance
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
Learning Objectives
Explain how the adaptive immune system is capable of recognizing so many different unique antigens with such a limited genome size
Outline the mechanisms that generally prevent the immune system from attacking the body
Describe why the immune system sometimes attacks the body anyways
Agenda 1: Review Goals of the Immune System
PAMPS are only expressed by ? And DAMPS are ? But both ?
PAMPS are only expressed by non-self and DAMPS are endogenous, but both activate innate immunity via PRRs
Agenda 1: Review Goals of the Immune System
NK cell inhibitory ligands, especially what, are mostly expressed by what? What can some pathogens do?
NK cell inhibitory ligands, especially MHC-I, are mostly expressed by self
some pathogens can express inhibitory ligands to avoid detection
Agenda 1: Review Goals of the Immune System
What do lymphocytes mostly and sometimes recognize?
lymphocytes mostly recognize non-self antigens
but sometimes, they recognize self
Agenda 1: Review Goals of the Immune System
What do the PAMPs and DAMPs tell the innate system?
PAMPs and DAMPs tell the innate immune system when something is dangerous
Agenda 1: Review Goals of the Immune System
What does the adaptive immune system recognize and doesn’t?
adaptive immune system recognizes antigen but doesn’t know where it comes from (variable region doesn’t know what the function should be, and only what antigen it is)
so it has to trust the innate system, but what if the innate system is wrong?
Agenda 1: Review Goals of the Immune System
But, there is a lot of room for error, which is how we get….
pathogen evasion, cancer, autoimmunity, chronic inflammation, organ transplant rejection, etc.
Agenda 2: Immune Diversity: Antigen Receptor Generation
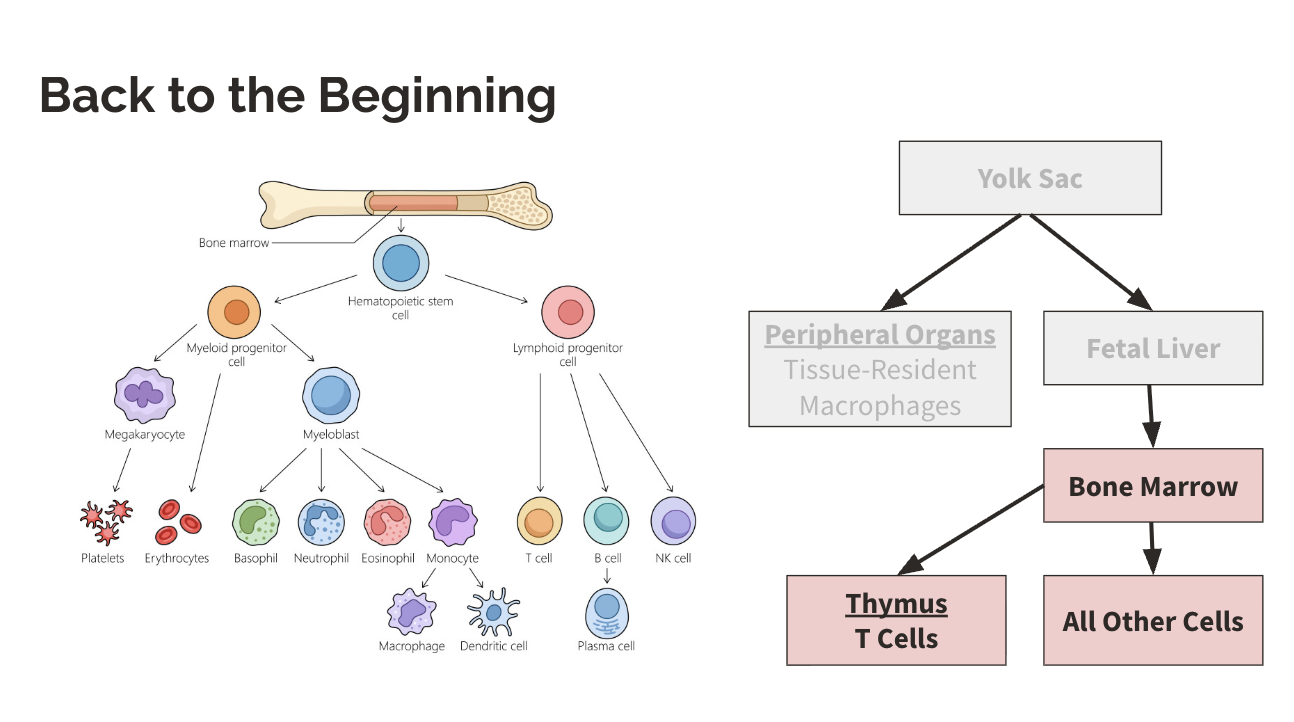
Where do B cells stay to be educated?
bone marrow
Agenda 2: Immune Diversity: Antigen Receptor Generation
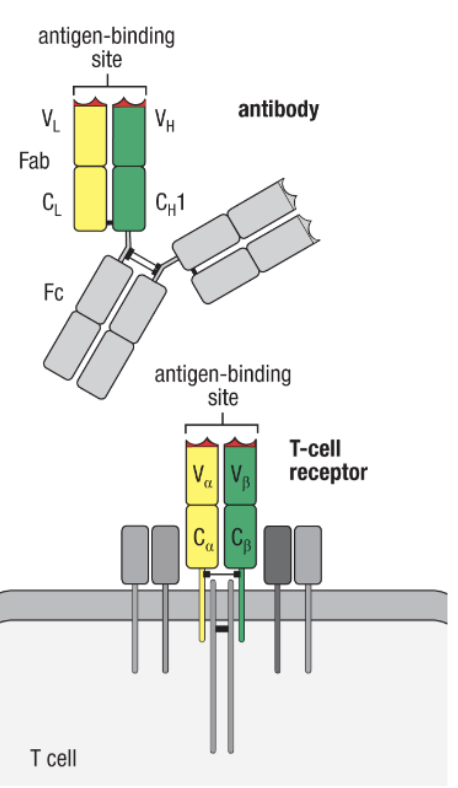
What cells have Fc region receptors?
B cells have, but T cells do not
Agenda 2: Immune Diversity: Antigen Receptor Generation
B and T cells are what? and can only be what? How many can be recognized?
B and T cells are antigen-specific and can only be activated by their specific antigen
estimated >108 different antigens recognized
Agenda 2: Immune Diversity: Antigen Receptor Generation
What question does recognizing >108 different antigens bring up?
if each antigen receptor was encoded by its own gene, there wouldn’t be enough space in the genome to encode the number or unique antigen receptors we actually observe
Agenda 2: Immune Diversity: Antigen Receptor Generation
soooo how do we get them all?
reserve a limited part of the genome for antigen receptors, but rearrange that part for many permutations
basically means to recombine parts of the genome
Agenda 2: Immune Diversity: Antigen Receptor Generation
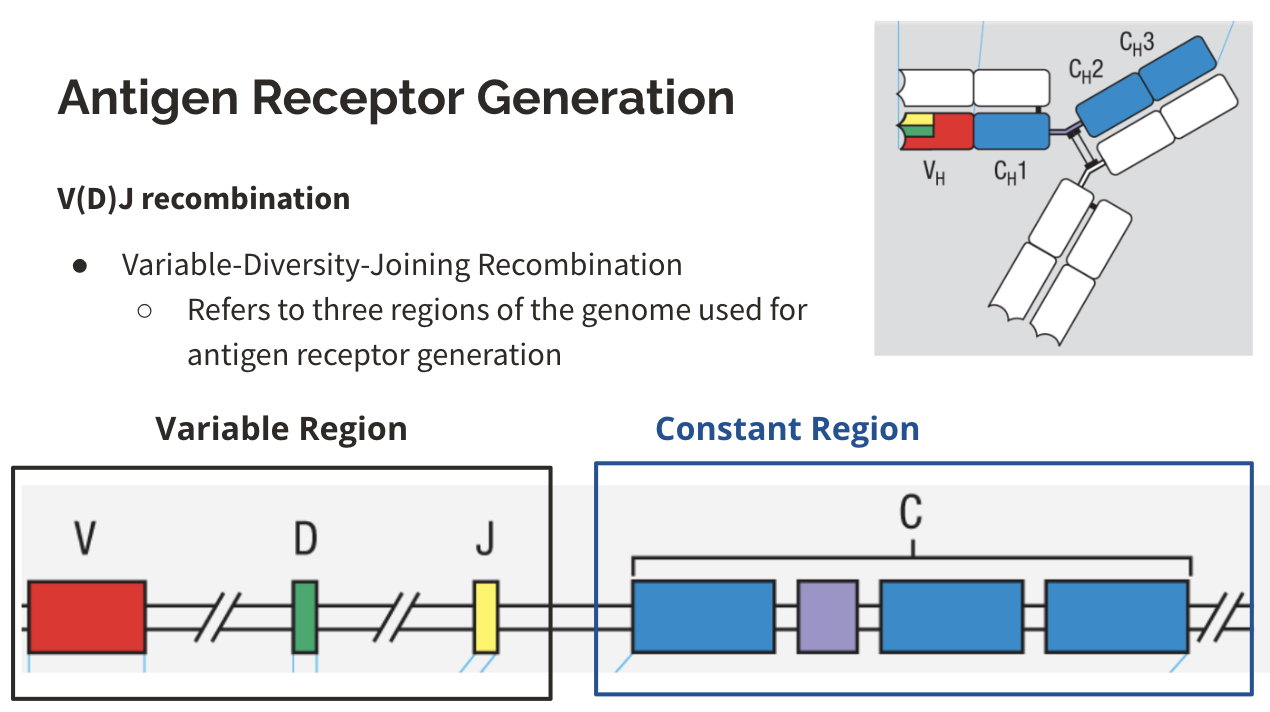
What part will change for an antigen receptor generation?
V(D)J recombination
variable-diversity-joining recombination, which refers to three regions of the genome used for antigen receptor generation
Agenda 2: Immune Diversity: Antigen Receptor Generation
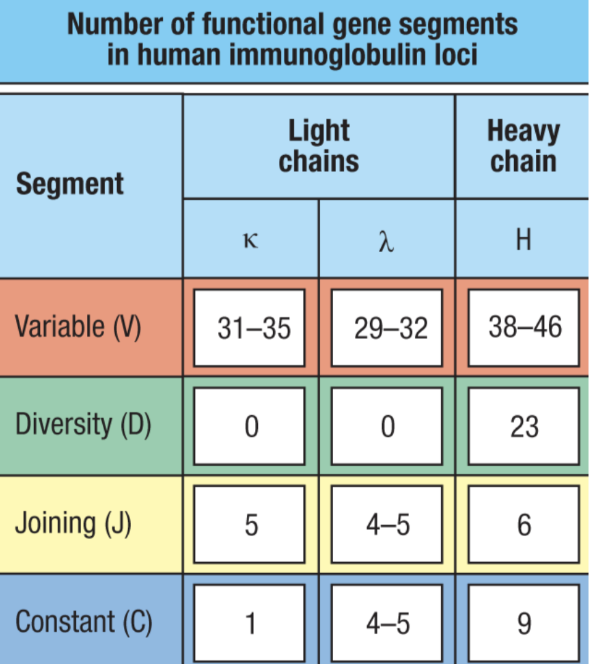
What is one way V(D)J Recombination occurs?
combinatorial diversity
many functional gene segments for V, D, and J regions
randomly remove portions of each region and recombine V, D, and J segments → leading to many, many possible permutations
different combinations oc chains (𝜅 or 𝛌 in BCRs and α/ꞵ or 𝛾/δ in TCRs)
Agenda 2: Immune Diversity: Antigen Receptor Generation
What is another way V(D)J Recombination occurs?
junctional diversity
some nucleotides are added at the ends of segments when they are joined together
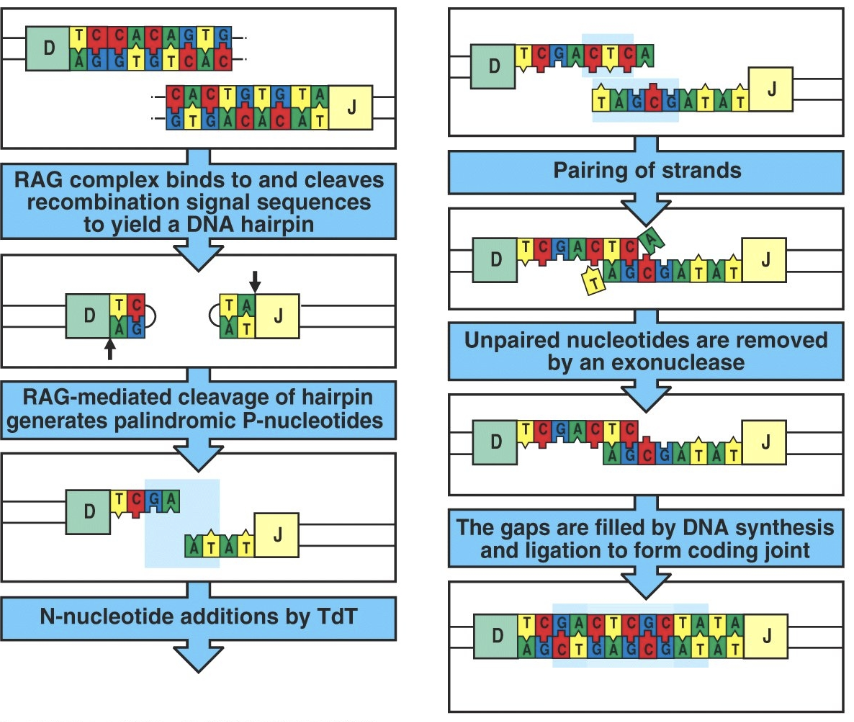
Agenda 2: Immune Diversity: Antigen Receptor Generation
What is another way that results in antigen receptor diversity?
affinity maturation: gives B cell diversity across one’s lifespan
B cells increase the affinity of their antibodies to antigens over time
especially upon secondary exposures & immunizations
somatic hypermutation: somatic gene rearrangement of the variable (antigen-binding) region
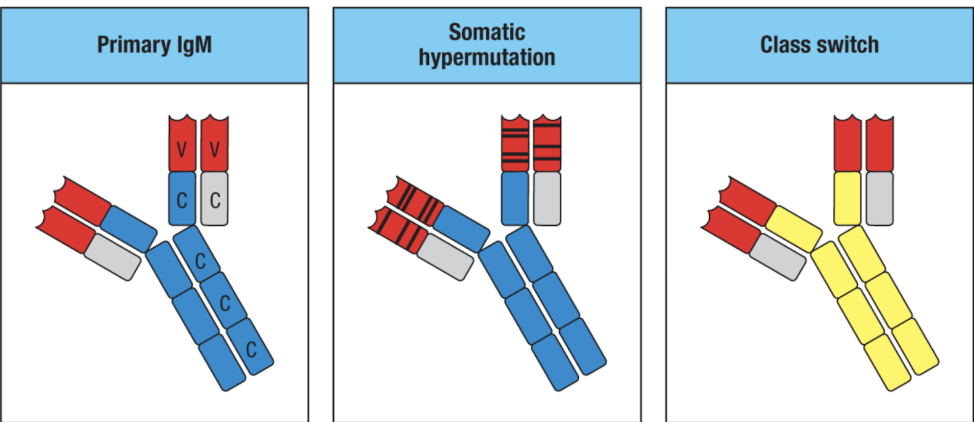
Agenda 3: Tolerance Mechanisms
What does tolerance mechanism mean?
the immune system is tolerating an antigen (it is not reacting to that antigen)
Agenda 3: Tolerance Mechanisms
What are the two types of tolerance mechanisms?
central tolerance: negative selection (“clonal deletion”) of self-reactive lymphocytes in central lymphoid organs during development
T cells: thymus
B cells: bone marrow
peripheral tolerance: various mechanisms for deleting or inactivating self-reactive lymphocytes outside of central lymphoid organs across the lifespan
Agenda 3: Tolerance Mechanisms
What happens in central tolerance in B cells? What are successful and unsuccessful tests? What is receptor editing? What is clonal deletion?
immature lymphocytes test their antigen receptors in the bone marrow during development
successful BCRs do not bind to common self antigen (ubiquitously common expressed antigens like MHC-I and housekeeping proteins) in the bone marrow environment
this does not include all self antigens in the body, so even if it passes this first test, it has to do another test because it might be reactive to antigens outside the bone marrow
unsuccessful BCRs rearrange their receptor (receptor editing) one more time and try again before dying (because it took a lot of energy to do this in the first place)
clonal deletion is induced apoptosis of self-reactive lymphocytes
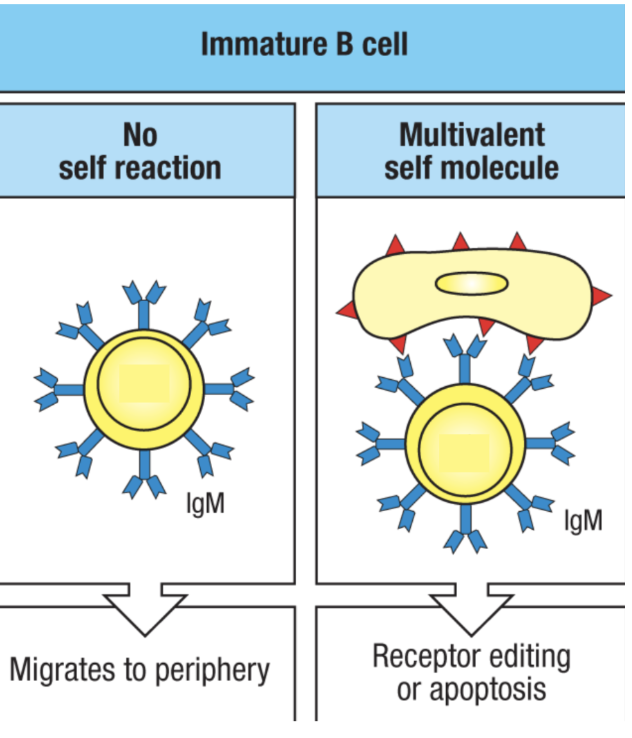
Agenda 3: Tolerance Mechanisms
What happens in peripheral tolerance in B cells?
immature B cells that leave the bone marrow become “transitional” and will still be negatively selected if they react to self antigen in periphery
apoptosis would be triggered
those that did not bind to an antigen becomes a mature B cell developmentally (but not mature as in it is activated because it is still a naive B cell)
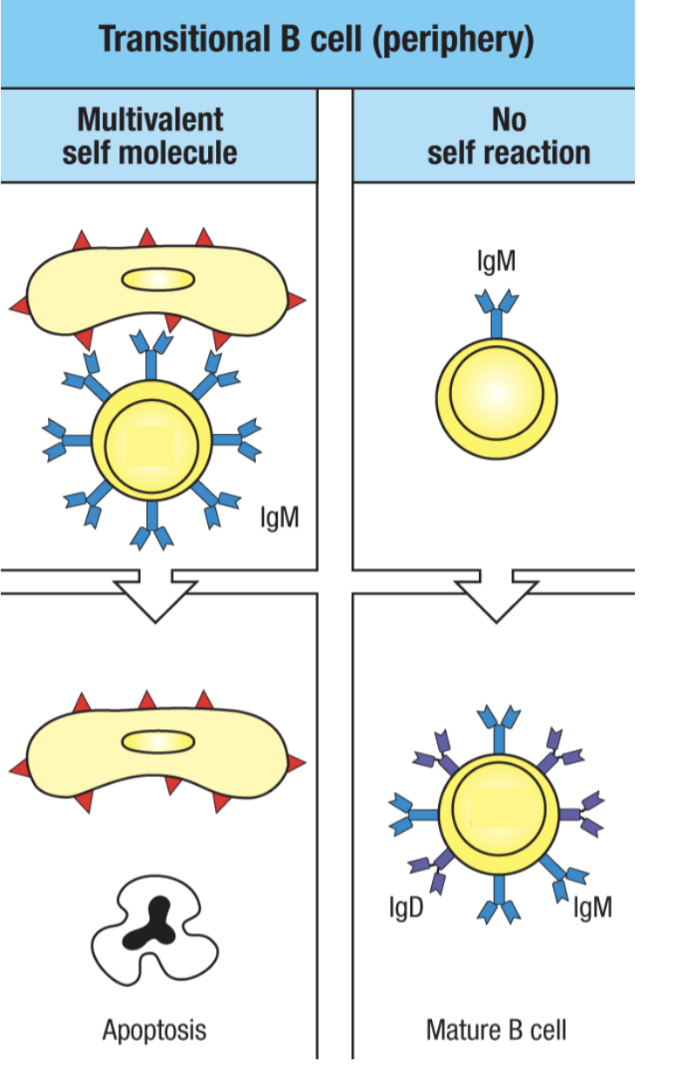
Agenda 3: Tolerance Mechanisms
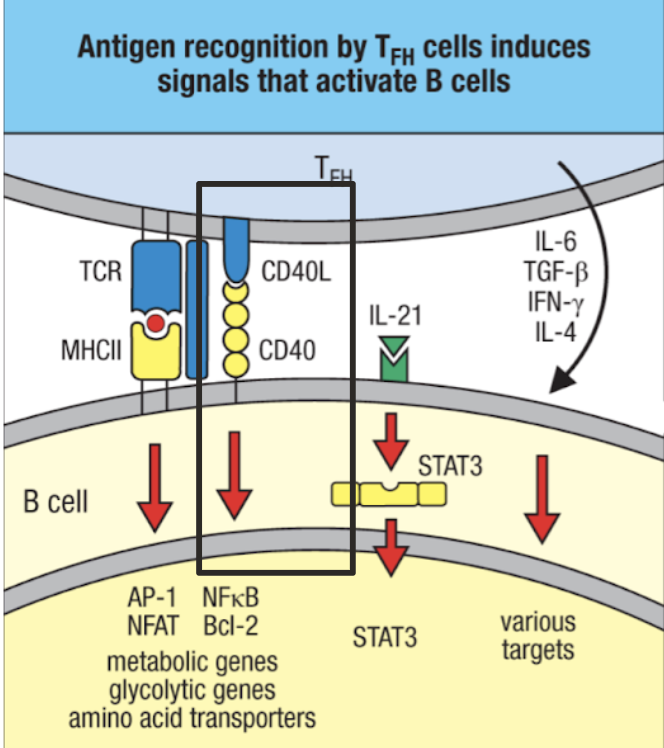
In peripheral tolerance B cells, how do most B cells must be activated by? Without activation of this, what can happen?
most B cells must still be activated by T cells, which undergo their own central and peripheral tolerance
antigen presentation without TFH costimulation leads to anergy (inactivation)
TFH won’t give costimulation if it has been inactivated by APC (generally because antigen is “self”)
Agenda 3: Tolerance Mechanisms
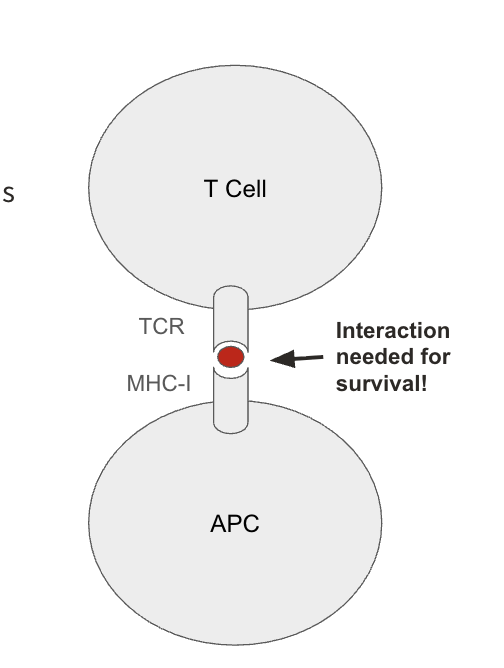
What is a successful and unsuccessful central tolerance in T cells?
successful TCRs should bind to non-self antigens loaded on MHC
specialized transcription factors in the thymus induces promiscuous (random) expression of endogenous antigens from all over the body (allows the T cell to check its TCR against the rest of the body without having it leave to the rest of the body to test reactivity)
sterile environment = no presence of non-self antigen
unsuccessful TCRs bind to self-antigens loaded on MHC or fail to bind to MHC
Agenda 3: Tolerance Mechanisms
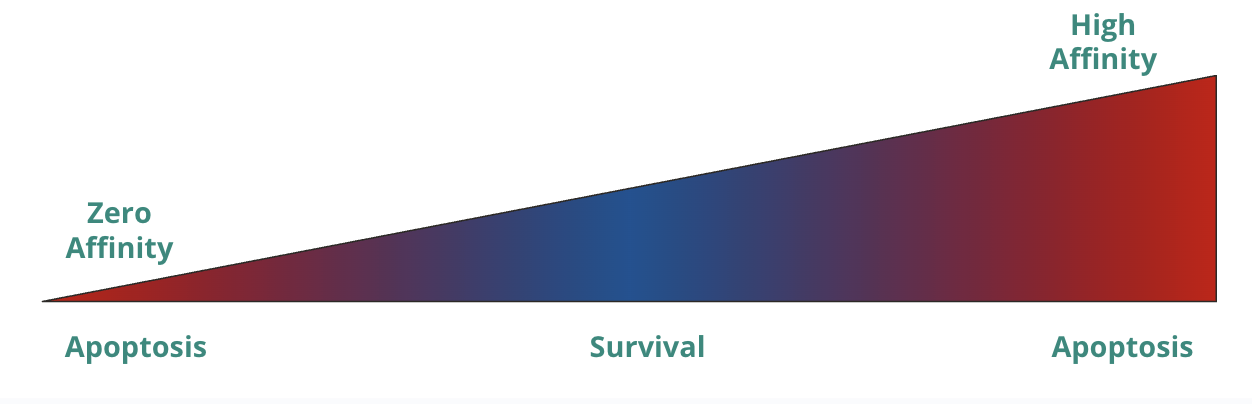
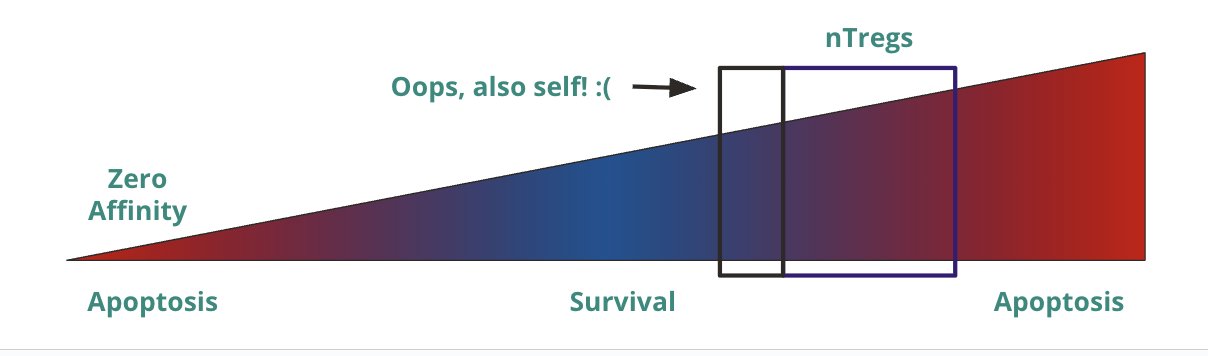
T cell activation is a spectrum based on what? What is this hypothesis called and mean?
t cell activation is a spectrum based on affinity
affinity hypothesis
no interaction (zero affinity) → apoptosis (receptor is so bad that it can’t even bind to MHC, so it is useless)
low/medium affinity → survival (able to bind MHC but not too reactive to self)
high affinity → apoptosis (reactive to self)
Agenda 3: Tolerance Mechanisms
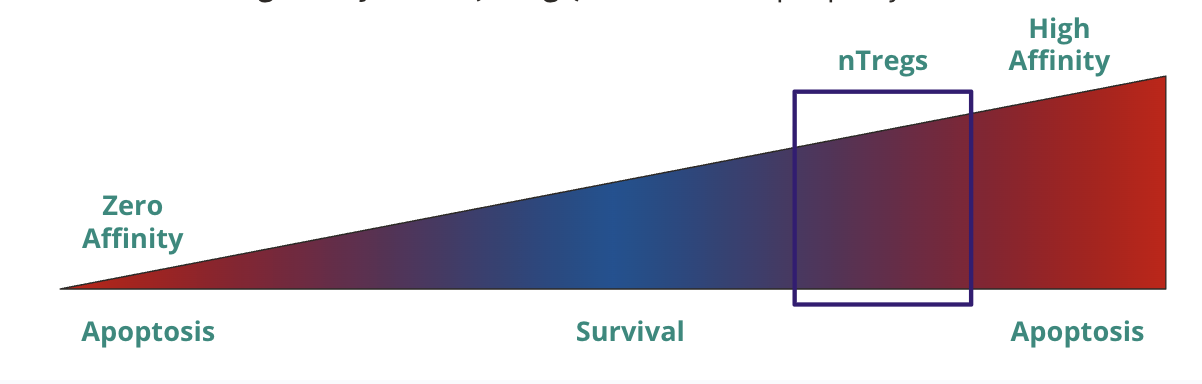
If T cell activation is a spectrum, how “low” is too low, and how “high” is too high?
low → unable to get strong enough signal for survival
high → T cells with intermediate/high activation become tolergenic/anti-inflammatory natural regulatory T cells (nTregs) that act in the periphery
Agenda 3: Tolerance Mechanisms
What are the two types of T cells in peripheral tolerance in T cells?
natural regulatory T cells (nTregs): induced in thymus when TCR is likely reactive to self-antigen
induced regulatory T cells (iTregs): induced in the periphery when APCs signal to CD4+ T cells that an antigen is self and/or healthy (like from your microbiome)
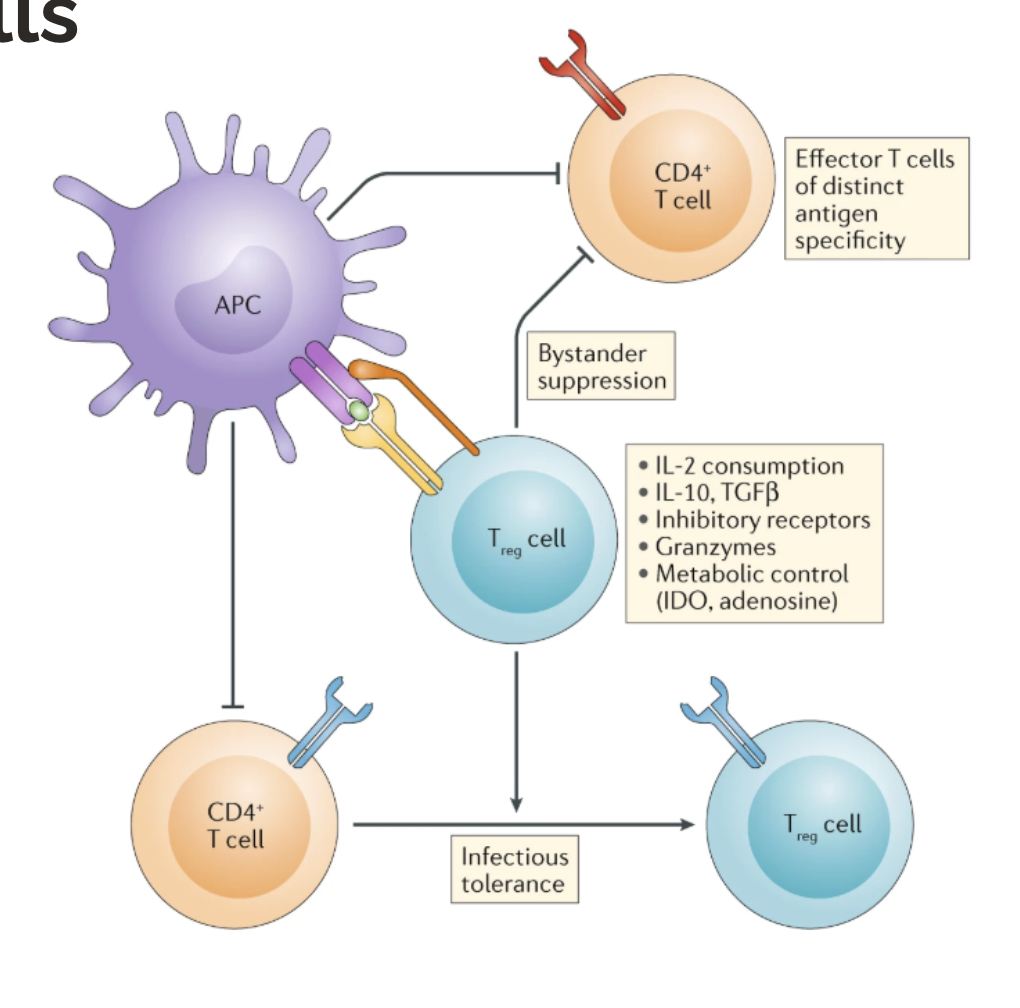
Agenda 3: Tolerance Mechanisms
T cell activation requires 3 signals. Without costimulation, what happens?
absence of costimulation implies self-antigen (no PRR signaling) → APCs may induce anergy (inactivate) T cells
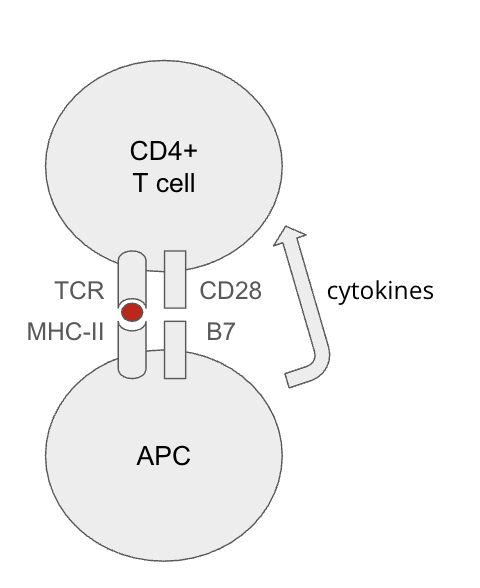
Agenda 3: Tolerance Mechanisms
What are checkpoint molecules?
activated T cells express inhibitory molecules
CTLA-4 (on the T cell’s surface): an agonist of B7 molecules (on APC, and thus, blocks CD28 signaling)
PD-1 (on the T cell’s surface): binds to PD-L1 and PD-L2 (on the surface of many things)
PD-L1 is expressed by wide variety of cells
PD-L2 is expressed by antigen presenting cells during inflammation
negative feedback loop is prevented from overactivation of T cells/immune system
exploited by pathogens and cancer cells
checkpoint inhibitors are a common and effective cancer treatment (by shutting down CTLA-4 on cancer cells’ receptors, then T cells are able to stay and fight against the tumors)
Agenda 4: Breaking Tolerance
T cell activation is a spectrum, so the threshold of nTreg induction …
may be a little off, so there is escape of self-reactive T cells
but this still requires PAMPs/DAMPs for innate activation (peripheral tolerance), but DAMPs can come from tissue damage and some self antigens can activate TLRs
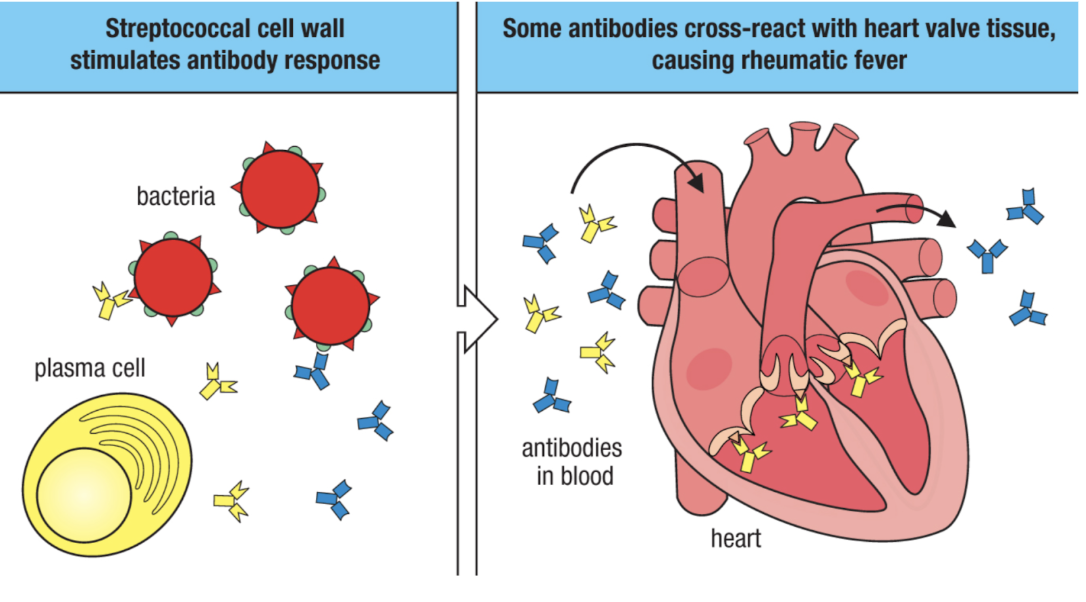
Agenda 4: Breaking Tolerance
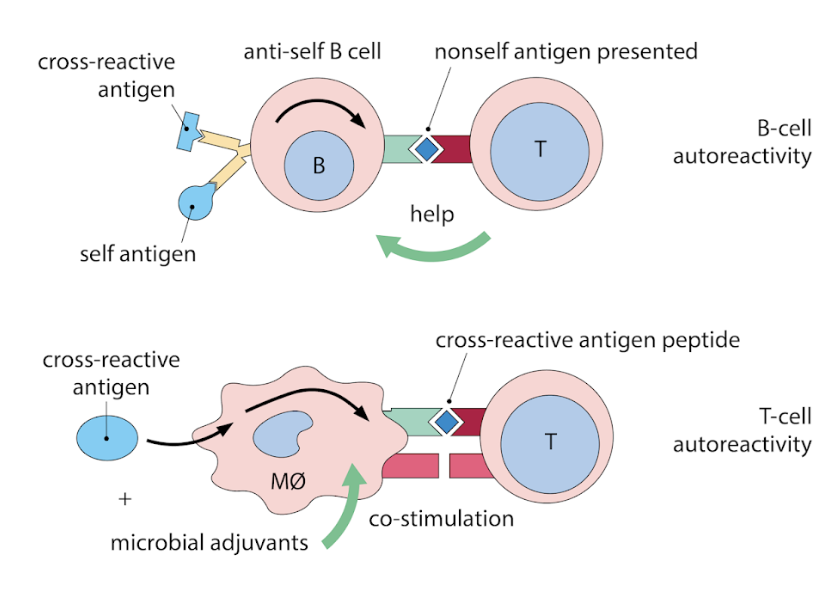
What is molecular mimicry?
molecular mimicry (cross reactivity)
non-self antigens resemble self antigens
infection with non-self (often a virus) induces an immune response that also targets similar looking self-antigens
basically, lymphocytes will target both the viral and self-antigens that look viral
Agenda 4: Breaking Tolerance
When can cross-reactivity occur?
during T cell activation or B cell activation
Agenda 4: Breaking Tolerance
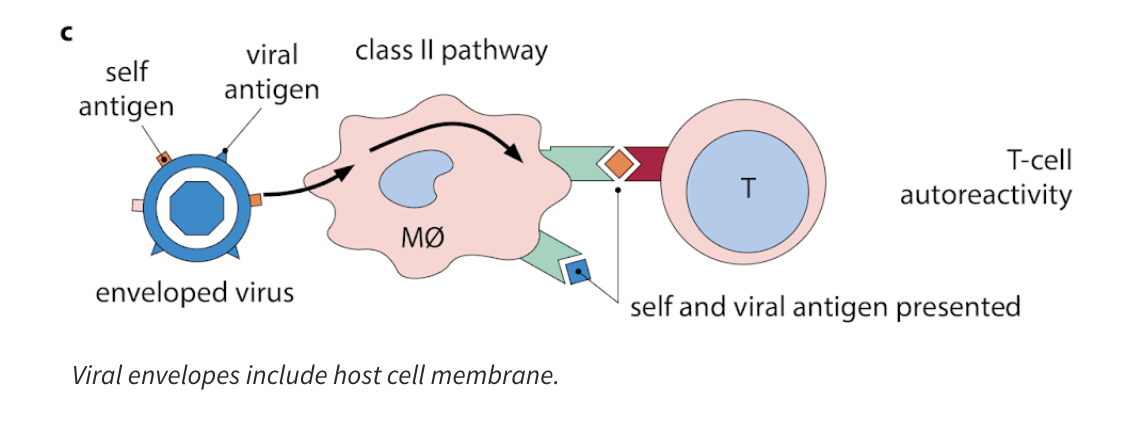
What can activated APCs do?
activated APC might present both self and non-self antigen at the same time
Agenda 4: Breaking Tolerance
What genetic mutations can this lead to?
improper central tolerance
poor checkpoint expression
improper T cell receptor signaling
poor regulatory T cell function
defects in apoptosis