Patho - Lecture 28 - Neuropathic Pain
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
What is the pathway on a sensation?
What are some examples of sensation?
stimulus —> receptor activation —> afferent transmission —> sensation
some examples include vision, hearing, fine touch (aka any of our 5 senses)
Why is pain a perception and not a sensation?
Explain what it means for pain to be subjective.
if pain was just a sensation, we would also only have the sensory component BUT pain includes both sensory + emotional interpretation
Therefore pain is subjective. if 2 people have the same injury, person 1 may find it more painful than person 2 depending on how they emotional perceive the injury
What is a nociceptor?
How are these receptors activated? and which fibers are used to relay this information to the brain?
What are the 3 types of nociceptors?
Which type of sensory nerves and which spinal tract detect mechanical stimuli?
Which type of sensory nerves and which spinal tract detect temperature and pain stimuli?
is a generic term for sensory receptors, but these receptors are specific for detecting pain
Nociceptors are activated by noxious stimuli and project to the spinal cord by unmyelinated (type C) or small diameter myelinated (type A‐delta) sensory axons.
3 types
thermal nociceptors — activated by heat/cold
mechanical nociceptors — activated by pulling, incisions, pinches
chemical nociceptors — activated by capsaicin from spicy foods or endogenous chemicals
Mechanical stimuli — Type A sensory fibers (not just delta but others too) and the Dorsal column tract
Temperature and Pain Stimuli — Type A delta AND Type C. These are through the spinothalamic tracts
What are the 3 dimensions of pain?
sensory — discriminative
perception of the pains location, intensity and duration
affective — motivational
emotional response to the pain
the urge to escape the unpleasant feeling (aka the pain)
cognitive — evaluative
interpretation and coping strategies related to pain
this may vary between individuals and cultures
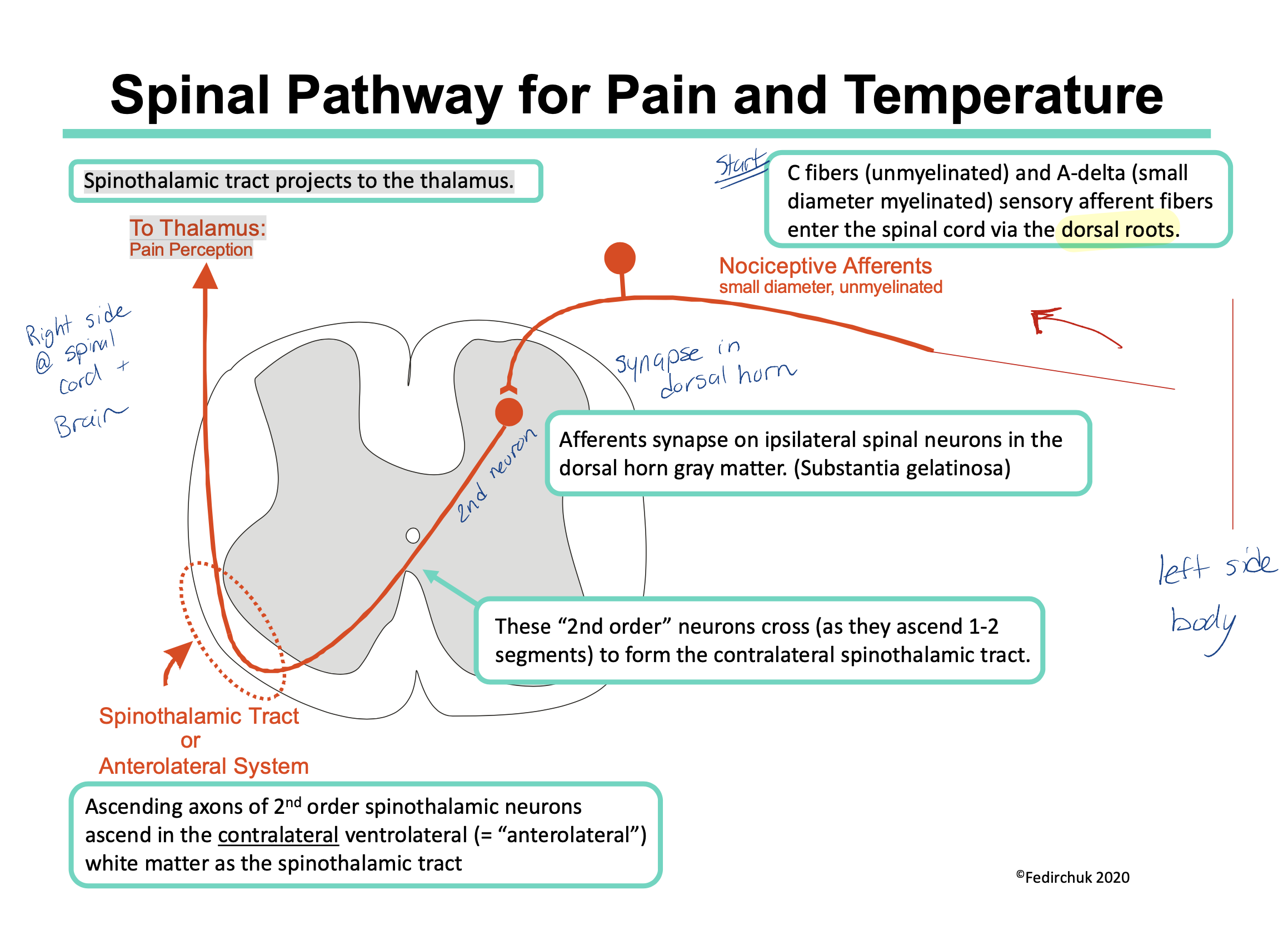
Describe the Spinal Pathway for Pain and Temperature
C fibers (unmyelinated) and Type A‐delta fibers (small diameter myelinated) enter the spinal cord via the dorsal roots
They synapse on same side they enter the spinal cord (ipsilateral) in the dorsal horn gray matter. (Substantia gelatinosa)
The 2nd neuron now crosses (during the length of 2 spinal segments) and the signal travels up on the opposite side of the body via the spinothalamic tract.
Spinothalamic tract projects to the thalamus.
thalamus to somatosensory cortex in brain
Note: a sensory input from the left side of the body will be processed in the right side of the brain (and vice versa)
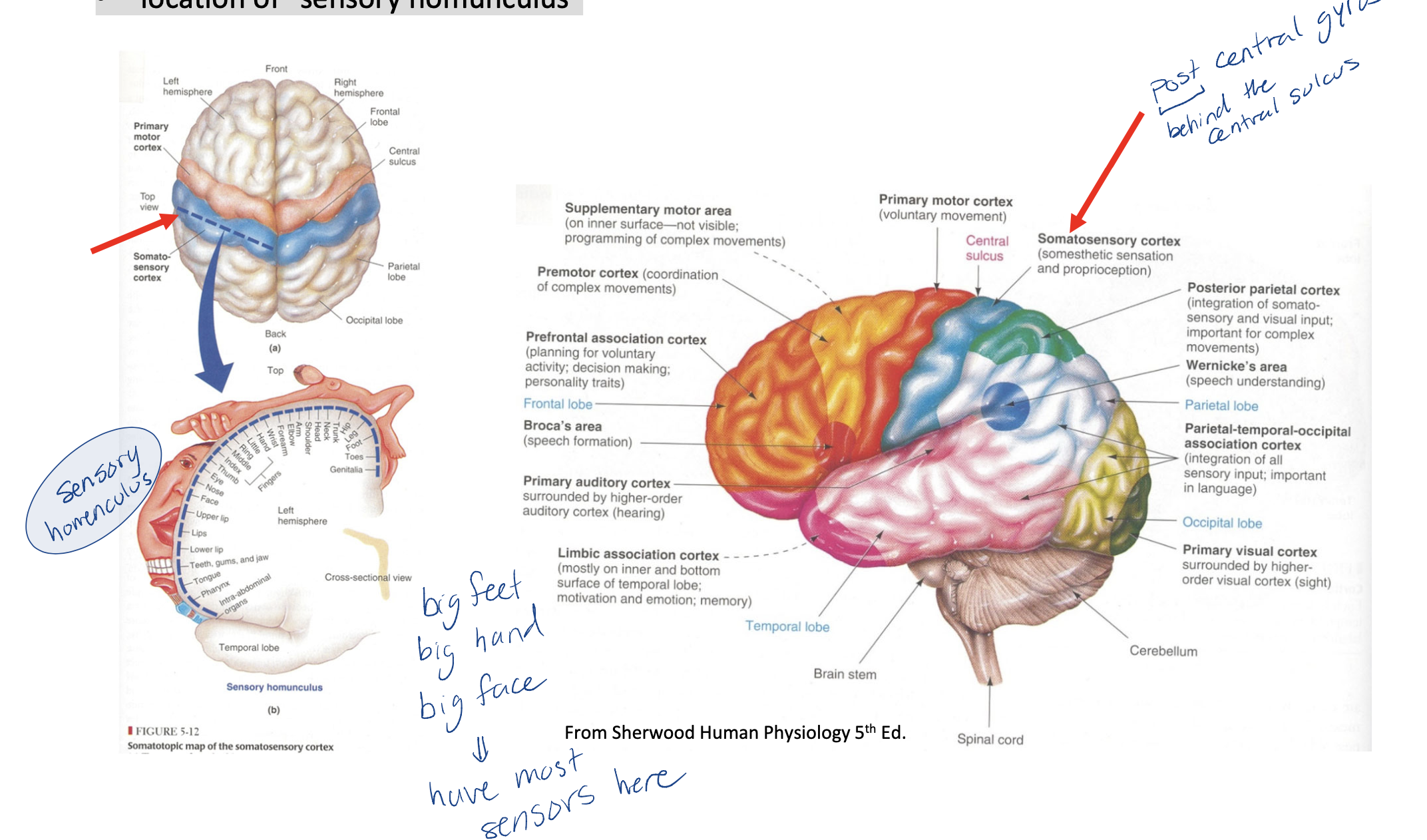
What lobe in the brain is the somatosensory cortex found in?
parietal lobe
somatosensory cortex (= primary sensory cortex = postcentral gyrus)
immediately behind the central sulcus (front of the parietal lobe)
location of “sensory homunculus”
What are some things that can not be explained by the classical pain pathway (6 things)
referred pain — pain being felt in a different area that is not the actual origin of the pain
trigeminal neuralgia — has a sudden onset, has no noxious stimuli and causes excruciating pain
fibromyalgia — no explanation for the widespread muscular pain
complex regional pain syndromes — e.g. nerve damage results in autonomic fibres activating nociceptive fibres —>pain (“causalgia”)
Phantom Limb Pain — still feeling pain in a limb even through the limb does not exist
Analgesia(feeling no pain) produced by acupuncture — you are getting pricked by many needles but don’t really feel the pain (mechanism not likely mediated by classic pain pathway)
Explain the steps of pain physiology. (immediate + soon after effects, long term effects)
immediate effects:
local burn
activates nocireceptors which causes action potential to form (first awareness of pain)
reddening of the injured area, swelling
soon after effects:
compounds (like histamine) are released to initiate anti-inflammatory response (the release of these compounds is triggered by the terminal ends of the nerves themselves)
we feel neurogenic pain. we see inflammation
“flare” stage = further redding and intense pain (hyperalgesia)
Long term effects:
Secondary hyperalgesia due to receptor sensitization and changes in CNS transmission
Pain sensation lingers beyond tissue damage
chance of prolonged pain changing this system
central synaptic plasticity and reorganization
When you feel nociceptive pain, what other factors may a person feel?
Over time... (minutes to days) there is the possibility of peripheral & central sensitization and plasticity leading to a state of....
Other factors:
Increased sympathetic activity
Fear and anxiety
Shallow breathing
leads to a state of chronic pain. Chronic pain can exist even if the actual tissue damage has been healed
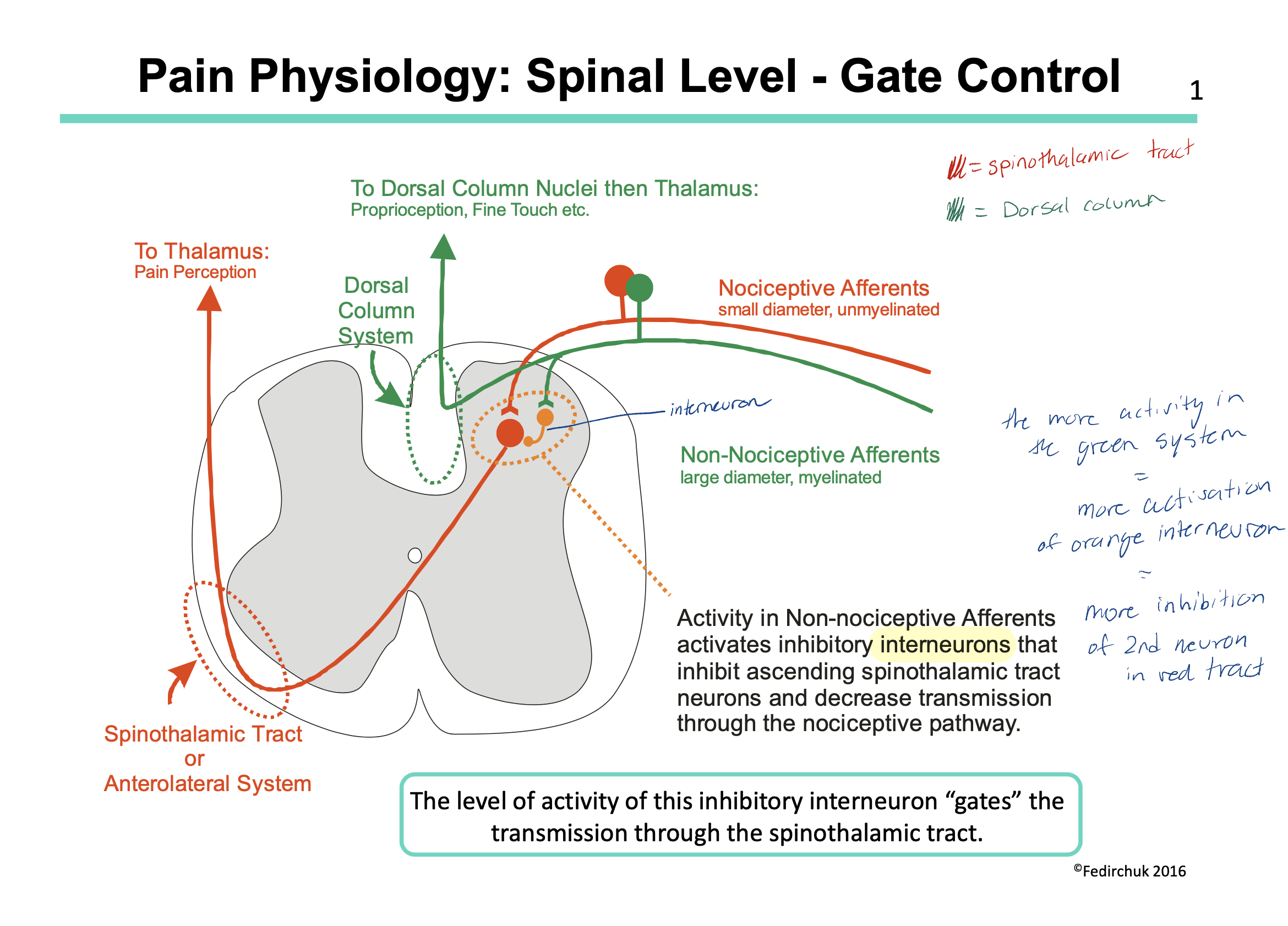
How can we control the level of pain we feel (at the spinal level)?
we have 2 types of afferent nerves entering our dorsal column: nociceptive afferent and NON-nociceptive afferent
the nociceptive afferent id the pain pathway we have been talking about this whole lecture (LOL) and work via the spinothalamic tract
the non-nociceptive afferent have interneurons that work via the dorsal column tract BUT also have inhibitory interneurons. These interneurons inhibit the spinothalamic pathway (aka they inhibit the pathway of pain)
in theory then, if we increase the amount of sensory input in our non-nociceptive afferents, we will increase the amount of interneurons = increase the inhibitory effect = decrease the amount of pain felt
this is actually how things like acupuncture, transcutaneous electrical nerve stimulation and clinical massage therapy work
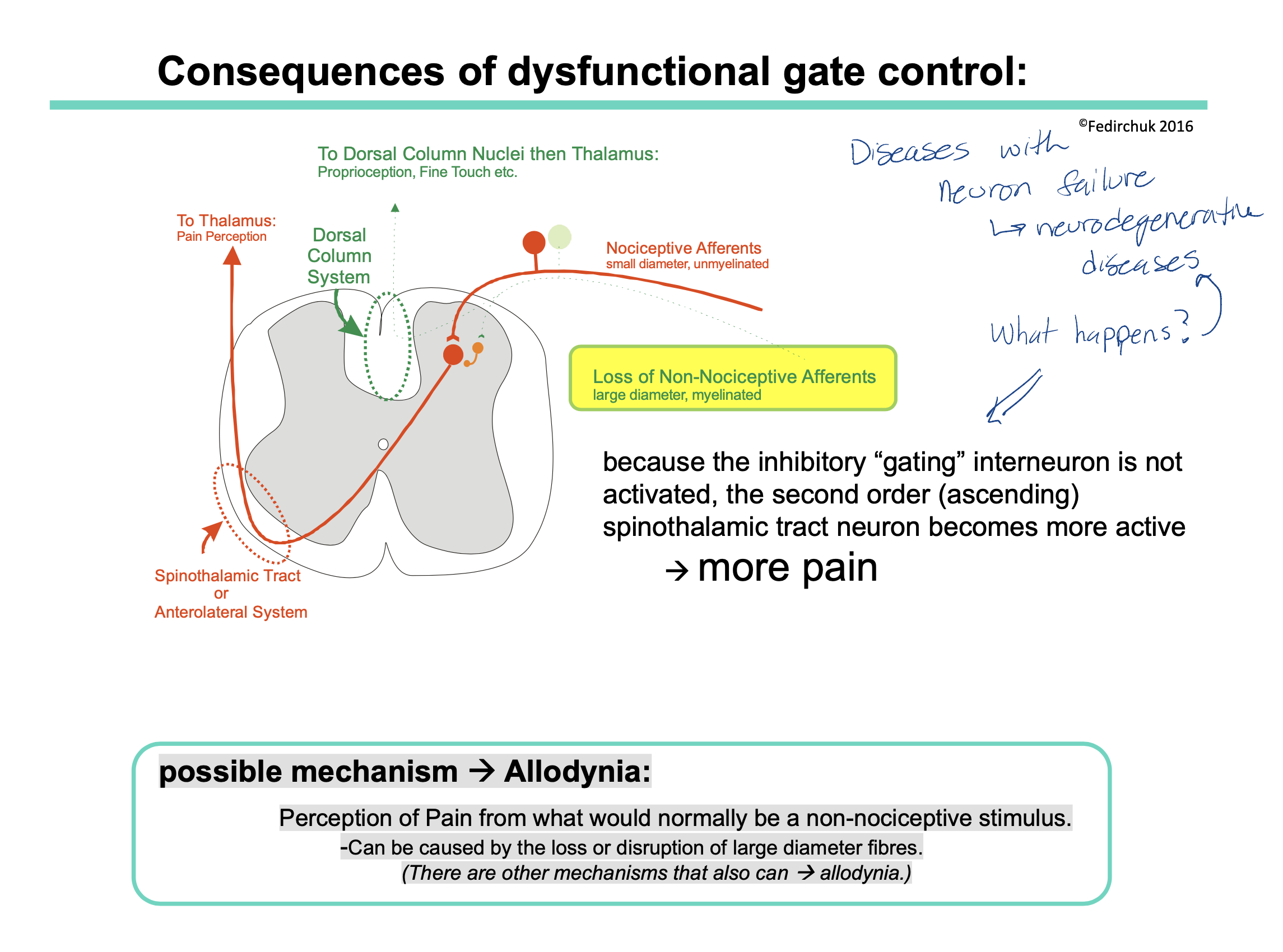
What is a consequence of dysfunctional gate control (for example, people with neurodegenerative diseases — where their neurons are affected)?
what is a possible mechanism for why we feel more pain?
If someone has a neurodegenerative disease, this could cause loss of certain neurons. This could be a problem if the neuron lost is the non-nociceptive neuron pathway.
Recall: the non-nociceptive neuron pathway sends mechanical signals to the brain via the dorsal column BUT also has the interneurons that inhibit the spinothalamic pathway (ie our pain pathway)
therefore if someone does not have the non-nocicpetive neuron pathway = less interneurons = less inhibition of the spinothalamic tract = more pain felt
possible mechanism = allodynia
allodynia = is a condition where a person perceives pain in response to a stimulus that is not typically painful
When non-nociceptive (aka large diameter fibers) are compromised, the normal inhibitory signals that prevent the perception of pain from non-nociceptive stimuli may be reduced.
As a result, stimuli that would typically be interpreted by the nervous system as non-painful (such as light touch or temperature changes) are now processed as painful signals.
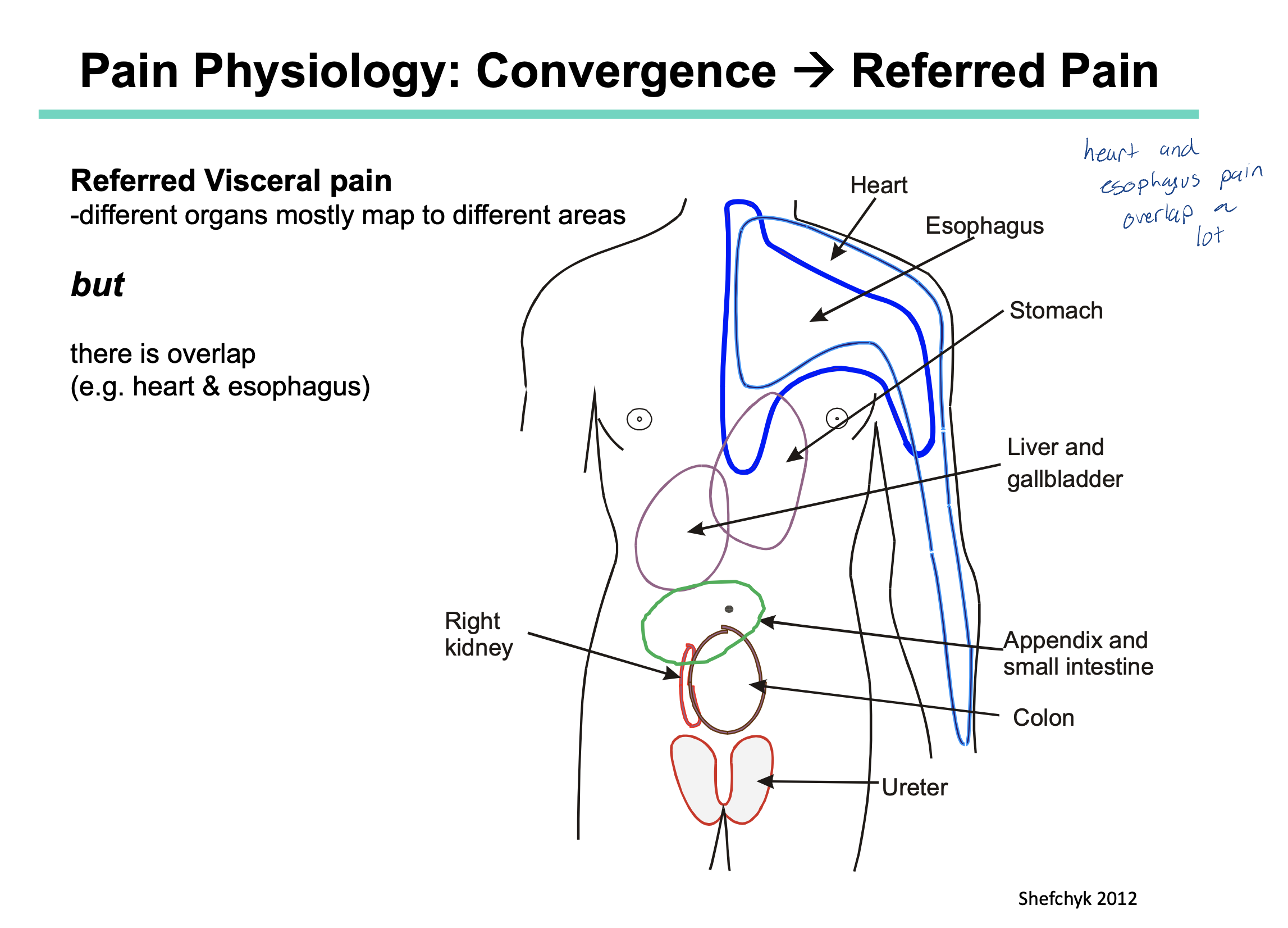
What is convergence?
What is referred pain?
How does convergence lead to referred pain?
Convergence is when 2 neurons synapse at the same postsynaptic neuron. (ie. 2 neurons converging onto 1)
Referred pain is when pain is felt in a area of the body that if different from where the pain’s actual origin is.
Nociceptive fibers from our skin, muscle and joints enter the spinal cord at the same point as nociceptive fibers from our organs AND they converge all at the same postsynaptic neuron. This information then travels to our brain and is interpreted using our sensory homunculus.
recall: our sensory homunculus has more receptors present on areas such as the head, limbs, feet etc (ie places where we know what pain feels like) and less receptors (in comparison) on our organs
this is why the brain might interpret heart pain as being a sore arm
we also have areas that may overlap with other organs. Example) pain in the stomach could also be interpreted as pain in the esophagus (because these two areas overlap slightly — refer to image)
what does modulation of the pain system mean?
what are the sites of modulation?
Modulation of the pain system refers to the complex regulatory processes that can enhance or suppress the transmission of pain signals within the nervous system
sites of modulation:
sensory modulation (gate control in the spinal cord)
descending modulation
serotonergic
noradrenergic
dopaminergic
endogenous opioid antinociception system
endogenous cannabinoid antinociception system
What are the 3 monoaminergic descending systems of modulation?
How do these systems work (in general)?
Which systems increase pain? Which decrease pain?
3 systems:
dopaminergic
noradrenergic
serotonergic
The periaqueductal gray matter (PAG) = special area in the midbrain that regulating pain systems. When the PAG is activated, it activated the pons and medulla which send descending projections down the spinal cord which can alter sensory processing (therefore change how we perceive pain).
Dopaminergic and noradrenergic systems decrease pain
Serotonin pathways can increase OR decrease pain
Which monoamines play a role in regulating our mood?
What is the monoamine hypothesis?
What are 3 things that decreased serotonin levels can lead to?
Why are antidepressants considered a treatment option for pain?
Which drugs inhibit reuptake of serotonin and noradrenaline?
Which drugs are more effective for neuropathic pain?
serotonin and noradrenaline
The monoamine hypothesis suggests the imbalances in neurotransmitters (like serotonin and noradrenaline) can lead to dysfunctional neuron signaling which can impact our mood.
Decreased serotonin can lead to:
depression
anxiety disorders
trouble reducing/suppressing anger
Antidepressants (which increase the amount of neurotransmitters) help to correct these neurotransmitter imbalances and re-elevate our mood. Since the neurotransmitters are involved in both pain and mood modulation, antidepressants can be used to treat both depression + pain.
Drugs:
for inhibiting reuptake of serotonin and noradrenaline —> TCA = tricyclic antidepressants
For neuropathic pain —> SSRI = selective serotonin reuptake inhibitors
How does the endogenous opioid system work for pain modulation?
What are the different types of opioid receptors?
What happens to this pathway when you have an overdose with EXOGENOUS opioids (ex heroine, fentanyl)?
The endogenous opioid system works for pain modulation when the periaqueductal grey matter(PAG) is activated which releases naturally occurring substances, such as endorphins and enkephalins, that bind to opioid receptors in the CNS. Activation of these receptors inhibits the transmission of pain signals by inhibiting neurotransmitter release, leading to analgesic effects and the modulation of pain perception.
types of opioid receptors:
mu
kappa
delta
Overdose with exogenous opiods:
it leads to respiratory depression
it is treated with Naloxone (Narcan) = opioid receptor antagonist
How does the endogenous cannabinoid system work for pain modulation?
What are some other system effects that cannabinoids cause?
periaqueductal grey matter is stimulated and releases endogenous cannabinoids (ex. anandamide= AEA and 2 arachidonoylglycerol = 2-AG)
these endogenous compounds bind to the cannabinoid receptors (Cb1 and Cb2)
normally these receptors are upregulated in pain therefore by inhibiting them, we decrease the amount of pain felt
these compounds also help decrease inflammation caused my microglia and astrocytes in the CNS
other effects:
can lower blood pressure (via vasodilation)
slow heart rate
lower intraocular pressure
stimulate appetite
anti‐inflammatory
reduces pain
anti‐nausea
How can the gate control theory be activated without neurodegenerative diseases?
How can the endogenous descending pain pathways be activated without drugs?
the more area/large diameter you stimulate = more dorsal column afferent activity —> less transmission through the spinothalamic system
• e.g.’s acupuncture, exercise, massage, TENS
more activation of endogenous monoaminergic, opioid, cannabinoid systems.
i.e. ↑ activation —> ↓ Pain
e.g.’s exercise, meditation, yoga, Cognitive Behavioural Therapy (CBT)
What are some of the positive and negative symptoms that can occur when the spinothalamic tract (ie our pain tract) is damaged/dysfunctioned?
Positive symptoms:
paresthesias = abnormal sensations in the skin, such as tingling, numbness, or a "pins and needles" feeling.
spontaneous pain
increased sensation of pain (e.g. allodynia)
Negative symptoms:
analgesia (inability to detect nociceptive stimuli)
loss of temperature appreciation
may be relayed as “numbness” despite preserved large diameter sensory modalities.
What is the difference between nociceptive pain VS neuropathic pain?
Nociceptive pain, on the other hand, arises from actual or potential tissue damage and is a normal response to harmful stimuli, such as injury or inflammation
Neuropathic pain is caused by damage or dysfunction of the nervous system, leading to abnormal signaling and sensations, such as tingling or shooting pain.
What is neuropathic pain?
What is peripheral neuropathic pain vs central neuropathic pain?
neuropathic pain = Pain of neural origin
may be produced by dysfunction of peripheral or central pain circuitry
not due to prolonged external noxious stimulus
Peripheral = often produced by prolonged release of inflammatory compounds from peripheral nerves (i.e. neurogenic inflammation)
central = can follow lesions of the CNS (e.g. after stroke) = “thalamic pain syndrome”
What are the properties of neuropathic pain?
pain out of proportion to the actual tissue injury (eg burning, tingling) andy any signs of nerve injury detected during neurologic examination.
May be triggered by Surgery, Amputation, Diabetes, Drugs (e.g. chemotherapy), Herpes Zoster (Shingles), HIV/AIDS, MS, Spinal Injury, Stroke, Tumor, Arthritis (and other triggers)
some neuropathic pain responds to opioids (not all)
gabapentin & pregabalin are centrally acting agents used to treat neuropathic pain
List the 4 clinical pain syndromes.
allodynia
phantom limb pain
central pain syndrome
complex regional pain syndrome (CRPS)
What is allodynia?
Allodynia is a condition where a person perceives pain in response to a stimulus that is not typically painful.
can be produced by different mechanisms
e.g. dysfunction gate control, sprouting/enhanced activation in the dorsal horn, other central changes
What is phantom limb pain?
pain perceived as arising from an amputated limb (sometimes amputated years ago)
no actual nociceptor is activated, so it is due to altered central processing
What is central pain syndrome?
AKA —> central neuropathic pain’ = ‘thalamic pain syndrome’
pain that occurs due to damage to CNS (e.g. stroke, SCI, MS)
potential mechanisms include:
hyperexcitability in remaining ascending Spinothalamic tract neurons, disinhibition within the thalamus
What is complex regional pain syndrome (CRPS)?
Does pain get better over time?
Which division of the nervous system is involved?
It is a chronic pain condition that typically affects one limb, often after an injury or trauma.
pain often described as burning, sharp stabbing or stinging
a gentle touch may cause them severe pain (= allodynia)
may follow trauma (even mild), surgery, or medical conditions (e.g. post M.I.)
involved extremity is often warm, edematous, tender and motion is painful
Symptoms vary in severity and durations (weeks, months, years)
CRPS can be severe and is often difficult to treat (early treatment is important)
Pain overtime:
CRPS pain continues after the original injury has healed.
may get worse with time, may spread to other limbs
The SNS division is involved
temperature differences in the limb on opposite sides (blood flow)
abnormal sweating
swelling of the painful region, and beyond
What are the 2 types of complex regional pain syndrome (CRPS)?
CRPS 1 = Reflex Sympathetic Dystrophy (RSD)
pain without nerve damage (driven by sympathetic system)
CRPS 2 = Causalgia
pain after nerve injury
activity in sympathetic fibres —> activation of pain fibres at site of injury
What are the treatment options for complex regional pain syndrome (CRPS)?
NSAIDs, Opioids
physiotherapy (keep moving – Gate control?)
neuropathic pain drugs (pregabalin, gabapentin)
List the 2 types of diffuse clinical pain syndromes.
myofascial pain syndrome
fibromyalgia
What is myofascial pain syndrome?
How do you treat this?
It’s a chronic pain disorder that is caused by certain trigger points—localized, hyperirritable spots within the muscles or fascia (connective tissue). These trigger points can cause pain and discomfort.
increased risk by repetitive movements, high levels of stress/anxiety
may (?) develop into fibromyalgia in some people
Treatments:
‐OTC analgesics, physiotherapy, massage therapy, acupuncture, antidepressant med’s (e.g. amitriptyline)
What is fibromyalgia?
How do you treat this?
its widespread pain, fatigue or sleep problems often due to emotional distress
disorder of pain processing —> lowered pain threshold (ie. feeling something as more painful than it should be = allodynia)
Treatments:
‐OTC analgesics, antidepressant med’s, ‘antilepileptic’ drugs (e.g. Gabapentin, pregabalin), physical therapy/exercise
What are the 2 general mechanisms in which we can reduce pain?
increase inhibition of pain pathways
or decrease excitation of pain pathways
How can we treat pain with drugs?
Narcotics / opioids:
E.g.’s Morphine, codeine, oxycodone
agonists of endogenous opioid system
decreases release of neurotransmitter at synapses in the pain system —> antinociceptive effects
Cannabinoids:
E.g. Medical Marijuana
agonists of endogenous cannabinoid system —> antinociceptive effects
Antiepileptics:
pregabalin (“Lyrica”); gabapentin (“Neurontin”)
increase concentration of GABA (—> increased inhibition)
also inhibits Vdep Ca2+ channels (e.g. at presynaptic terminals)
therefore they decrease neural excitability (hence utility as antiepileptics)
Antidepressants:
eg. amitriptyline (“Elavil”)
Noradrenaline and Serotonin reuptake blocker
increase efficacy of nociceptive modulatory systems
is a tricyclic antidepressant
eg.sertraline (“Zoloft”)
Selective Serotonin Reuptake Inhibitor (SSRI)
increases efficacy of serotonergic system (—>nociceptive modulation)
antidepressant
Analgesics:
e.g. NSAIDS
analgesics, and also anti‐inflammatory
(—>reduced peripheral activation of nociceptors)
Topical analgesics: lidocaine
applied topically
blocks Vdep Na+ channels needed for action potentials
”nerve block” of peripheral axons unmyelinated nociceptive are afferents most sensitive)
Topical analgesics: capsaicin
applied topically
is the chemical in chili peppers that increases “spicy‐ness”
acts on TRP (Transient Receptor Potential) receptors
anti‐nociceptive when applied topically (downregulation of TRP R’s?, depletion of substance P?, toxicity to unmyelinated fibres?)
How can we treat pain using NON-pharmacological approaches?
We want to ultimately activate the endogenous systems which help decrease our pain.
Use the “Gate Theory”:
TENS (Transcutaneous Electrical Nerve Stimulation);
Acupuncture
Massage ‐ activate large diameter, non‐nociceptive afferents = increased inhibition of ascending spinothalamic tract neurons
Exercise, Meditation: increases the activation endogenous opioid system (e.g. endorphins etc)