periodic trends, valence electrons, lewis dot structures, ionic/covalent/metallic bonding
1/21
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
parts of periodic table
groups: vertical columns, also called families
periods: horizontal rows
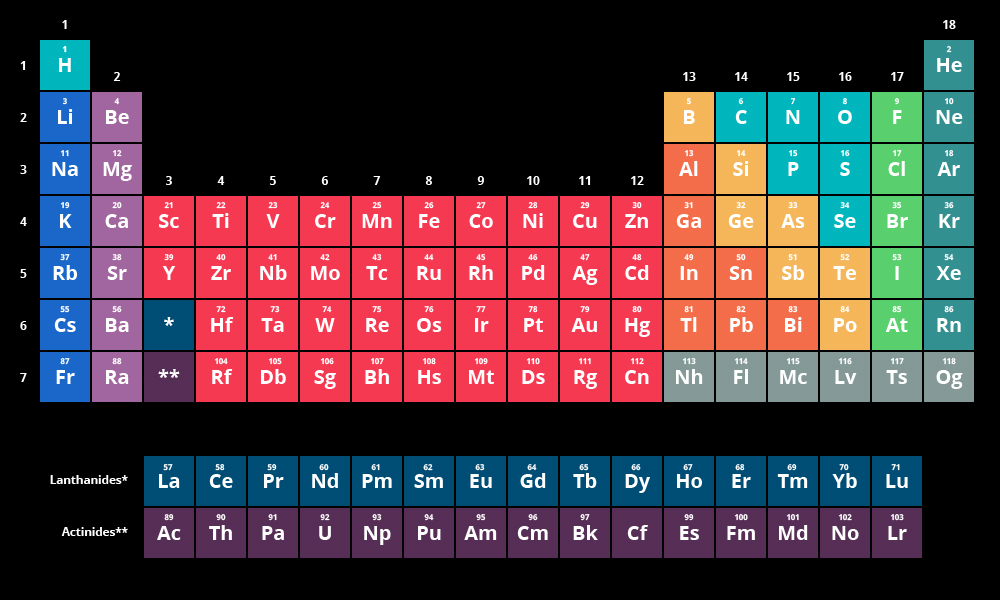
medium blue (1st column excluding hydrogen): alkali metals
light purple: alkaline earth metals
red: transition metals
orange: metals
yellow: metalloids
light blue: nonmetals
light green: halogens
darker green: noble gases
dark blue: lanthanides
dark purple: actinides
octet rule
atoms gain or lose electrons (react) to get a full valence shell, which is 8 electrons in most atoms and 2 for hydrogen and helium
noble gases tend not to react because they already have full valence shells and don’t need any more/fewer electrons
electron configuration down a group
every atom in a group has the same number of valence electrons
this leads to a number of shared properties
trend: atomic radius
size of an atom
increases down a group
adding energy levels → shields outer electrons from attraction to nucleus (opposite charge), creating a weaker nuclear pull
decreases across a period
filling up same energy levels → more electrons added to shell + more protons in the nucleus = larger, more positive nucleus has increased pull on electron cloud, leading to a denser atom
trend: ionization energy
energy required to take an electron away from an atom
decreases down a group
more energy levels = weaker pull → easier to remove electrons from atoms with more energy levels
increases across a period
extra electrons + bigger, more positive nucleus = stronger nuclear pull → harder to remove electrons
trend: electronegativity
tendency of an atom to attract electrons in a chemical bond between atoms
decreases down a group
shielding from more energy levels creates weaker nuclear pull → harder to attract electrons
increases across a period
stronger nuclear pull makes it easier to attract electrons
think of octet rule: atoms with more valence electrons want to gain rather than lose - e.g. oxygen has six valence electrons so it’s easier to gain two than lose six
trend: electron affinity
energy released when gaining an electron
decreases going down a group
harder to gain electrons and thus less favorable
increases across a period
stronger pull makes it easier to gain an electron
ionic bonding definition
intramolecular (within molecules)
complete transfer of valence electrons
usually metals lose electrons, becoming cations
usually nonmetals gain electrons, becoming anions
electronegativity difference must be greater than 2.0
opposite charge between cat and anions leads to strong attraction - this is the ionic bond
ionic formula
write metal symbol first
determine the ionic charge of both
figure out the number of each using the least common multiple
i.e. cation with +2 and anion with +3 needs three cations and 2 anions
type 2 metals
pretty much overlap with transition metals
can have more than one ionic charge
Fe2+ and Fe3+ for example
written with roman numerals in parentheses that represent charge not quantity
Iron (II) means iron with 2+ charge not 2 iron atoms
naming ionic compounds
need to have the formula first
write name of cation first (use roman numerals if type 2)
sometimes suffix ‘ous’ is used for lower charge and ‘ic’ for higher charge for type 2 metals
add anion name with the ‘ide’ suffix
properties of ionic bonds
crystalline lattice structure - will shatter easily because of repulsion between like charges when ions get shifted
high melting and boiling points
very good electricity conductors but only when molten and aqueous (dissolved in water)
soluble in water (and dissociates in water)
covalent bonding definition
intramolecular bonding
sharing of valence electrons between atoms
usually 2 nonmetals
between atoms with similar pulls - less than 2.0 electronegativity difference
in each diagram, a line represents 2 electrons
single bond: sharing one pair of electrons (2 e-)
double bond: sharing two pairs (4 e-)
triple bond: sharing three pairs (6 e-)
formal method for determining formula
count the number of valence electrons (add or remove based on ionic charge)
determine the central atom → 1st in the formula name or least electronegative (never hydrogen)
form single bond between central atom and all others
distribute remainder of e- equally around the noncentral atoms then dump the rest on the central atom
check all octets - if needed replace pairs of electrons to form double or triple bonds
formal method addendums (🤪)
hydrogen only wants 2 e-
some elements can have more than 8 e- because of empty d sublevel
commonly P, S, Si, Cl
some only need 6 e- instead of a full octet
B and Be
put brackets [ ] around lewis dot structures of ions
naming covalent compounts
find formula first
write name of central/least electronegative atom first
only put numerical prefixes if there is more than one e.g. water is dihydrogen monoxide but CO@ is just carbon dioxide
for second atom, use numerical prefix and -ide suffix
properties of covalent molecules
brittle solids
usually low melting and boiling points - tend to be liquids and gases
poor heat and electricity conductors
‘like dissolves like’ - polar molecules only soluble in polar substances and likewise for nonpolar
polar vs. non-polar bonds
non-polar: equal-ish electronegativity (0 - 0.5 difference) leads to equal electron sharing
polar: less equal electronegativity (0.6 - 1.9) leads to unequal sharing of electrons
not fully positive and negative like ionic but one part is partially negative and one part is partially positive
can calculate bond type by calculating electronegativity difference - the bigger the difference, the more polar the bond
polar vs. non-polar molecule
IF molecule has non-polar bonds
the molecule is always non-polar
IF molecule has polar bonds
AND is symmetrical
the molecule is non-polar because molecule is more negative in all directions and thus equally negative
AND is asymmetrical
the molecule is polar
dipole
a molecule with two poles (regions with opposite charges)
dipole arrow represents this, with the cross at the more positive side and pointing to the more negative part
-| - - >
δ+ represents the more positive side and δ- represents the more negative side
molecular geometry
electron domain: bonds/bonding pairs (2 e- part of a covalent bond), lone pairs, and unpaired electrons
multiple bonds (double/triple) count as one domain
multiple bonds also count as one bond in the VSEPR model (valence shell electron pair repulsion theory)
lone pair electrons take up more space than covalent bonds so lone pairs ‘squash’ bond angles slightly (about 2.5 degrees)
solid wedge represents bond in front of the page and dashed wedge represents bond behind the page