Nucleic acids
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Nucleic Acids
macromolecules (DNA and RNA) that encode and transmit genetic information.
ho first isolated DNA and what did he call it?
Friedrich Miescher in 1868; he called it "nuclein."
DNA
A type of nucleic acid that carries hereditary genetic information in most organisms.
RNA
type of nucleic acid that plays a key role in protein synthesis and gene regulation.
general primary structure of nucleic acids
linear sequence of nucleotides joined by phosphodiester bonds in a nucleic acid strand.
Nucleotide
The building block of nucleic acids; consists of
a sugar
a phosphate group
and a nitrogenous base.
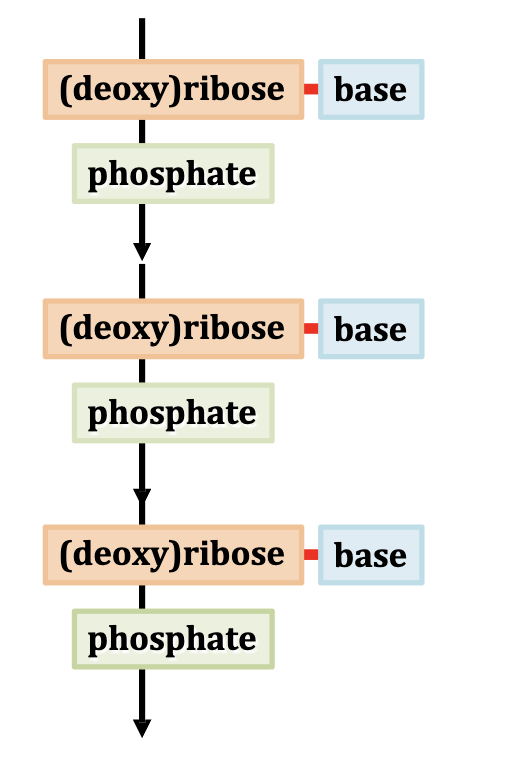
the sugar bc of having multiple oH groups bonds with both phosphate and base
back bone of nucleic acids
Repeating sugar-phosphate units linked by phosphodiester bonds.
Phosphodiester Bond
A covalent bond that links the 3′-OH of one sugar to the 5′-phosphate of the next, forming the nucleic acid backbone.
Nucleic acids have two kinds of pentose sugars:
B-D-ribose (the pentose sugar found in RNA)
B-D-2-deoxyribose (the pentose sugar found in DNA)
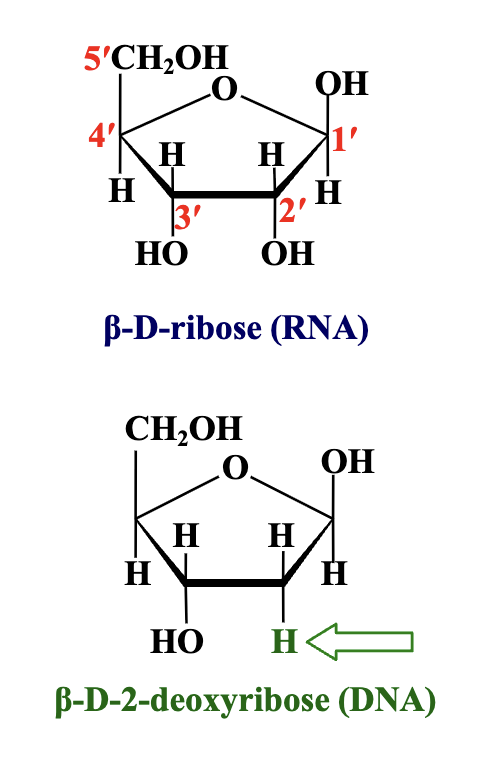
which pentose has a OH at the 2’ position?
The ribose (RNA) the pentose in DNA has a hydrogen instead of a hydroxyl making it deoxyribose
Why are sugar carbon atoms numbered with a prime (′) in nucleic acids?
To distinguish them from the carbon atoms in the nitrogenous bases
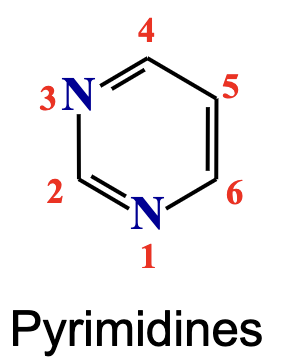
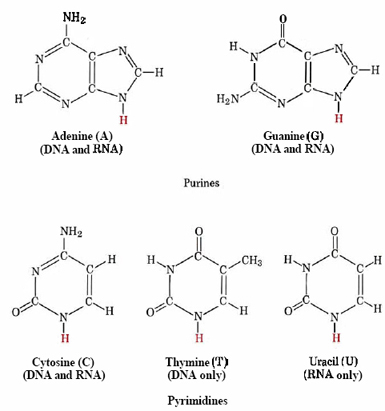
Pyrimidines
Single-ring nitrogenous bases found in nucleic acids; includes cytosine (C), thymine (T) (in DNA), and uracil (U) (in RNA).
Purines
Double-ring nitrogenous bases found in nucleic acids; includes adenine (A) and guanine (G).
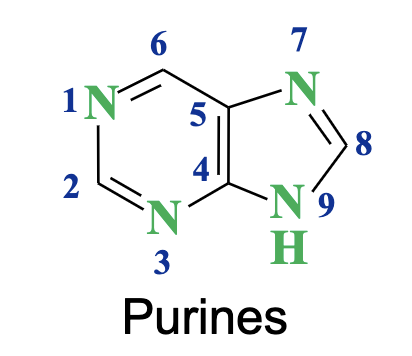
What are the complementary base pairs in DNA?
A→ T (2 H bonds) in RNA its U
G → C (3 H bonds) → stronger than AT
Heterocycle
Aromatic rings that contain non-carbon atoms such as nitrogen (N) within the ring; nucleic acid bases specifically contain nitrogen and carbon atoms only.
How Thymine and Uracil compare
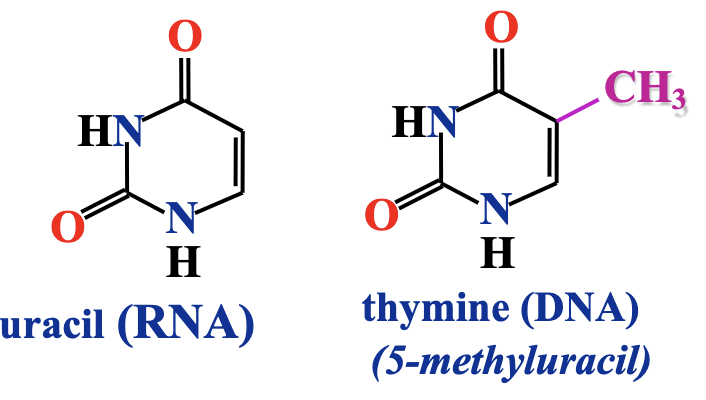
Both are pyrimidine bases with very similar structures; thymine is basically uracil with an extra methyl group (-CH₃) at the 5th carbon.
Thymine replaces uracil in DNA because the extra methyl group provides stability prevnting enzymatic degrdation.
Structure of all of the bases
Tautomers
Isomers of a molecule that differ by the position of a hydrogen atom and a double bond, often rapidly interconverting.
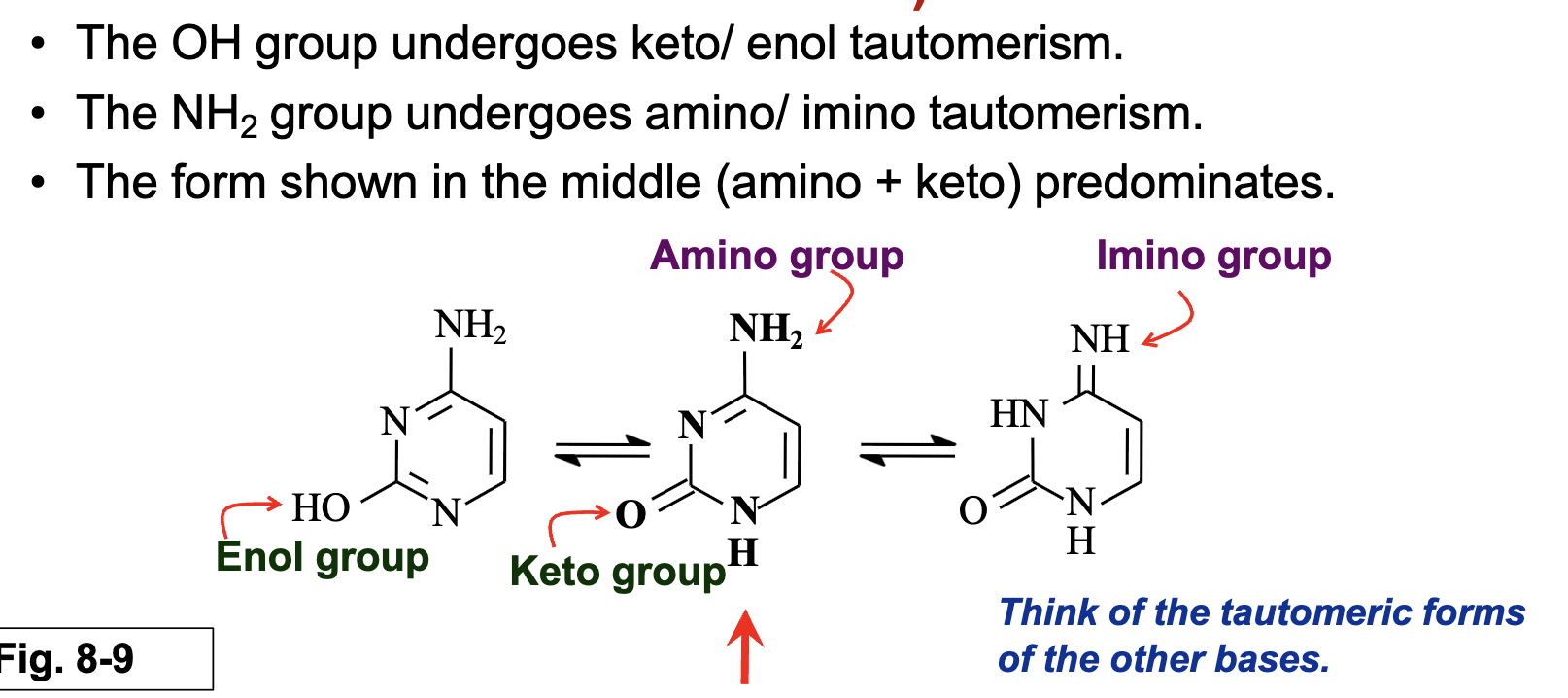
What tautomeric shifts occur in cytosine?
The OH group undergoes keto/enol tautomerism.
The NH₂ group undergoes amino/imino tautomerism.
amino + keto form predominantes
Nucleoside
A molecule consisting of a nitrogenous base linked to a sugar (ribose or deoxyribose) via a glycosidic (C–N) bond.
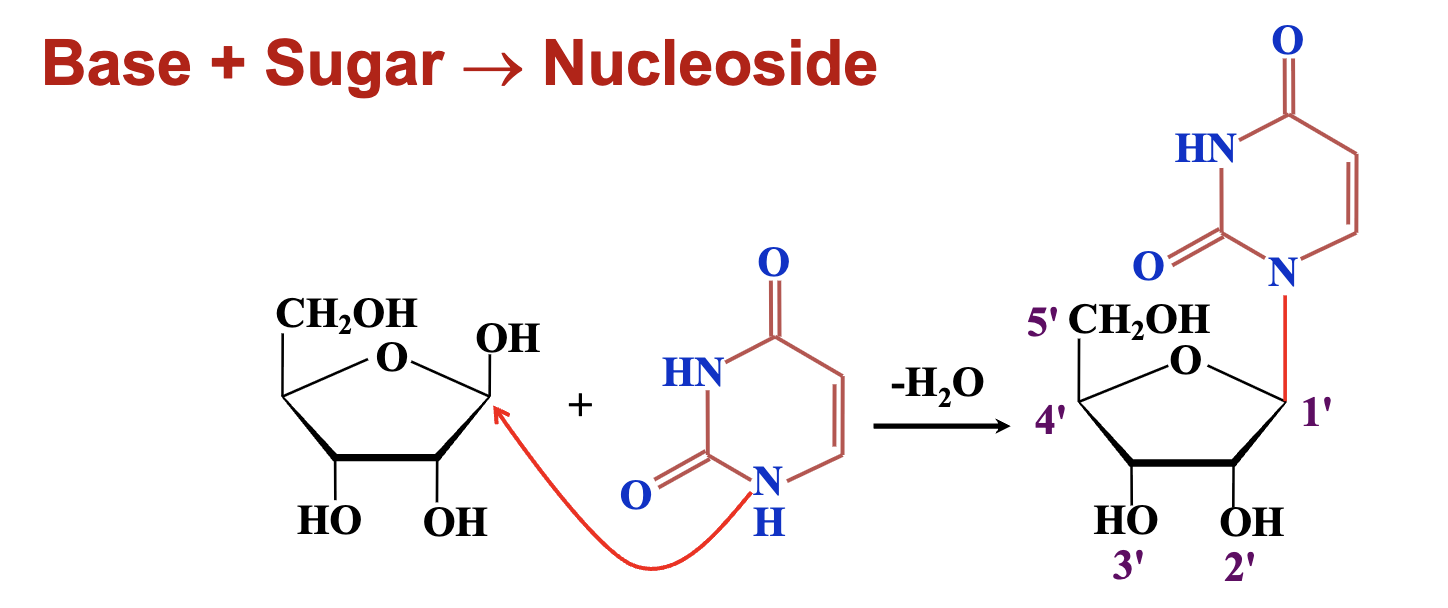
C–N linkage between the anomeric carbon of the sugar and a nitrogen atom of the base.
What is the difference between a nucleoside and a nucleotide?
A nucleoside is a base + sugar, while a nucleotide is a base + sugar + phosphate group.
To form a nucleotide or nucleoside the purine/pyrmimidine bases need to belinked to the sugars — how do they do that?
glycosylic bond (a C–N bond) links the nitrogen of the base to the sugar’s anomeric carbon.
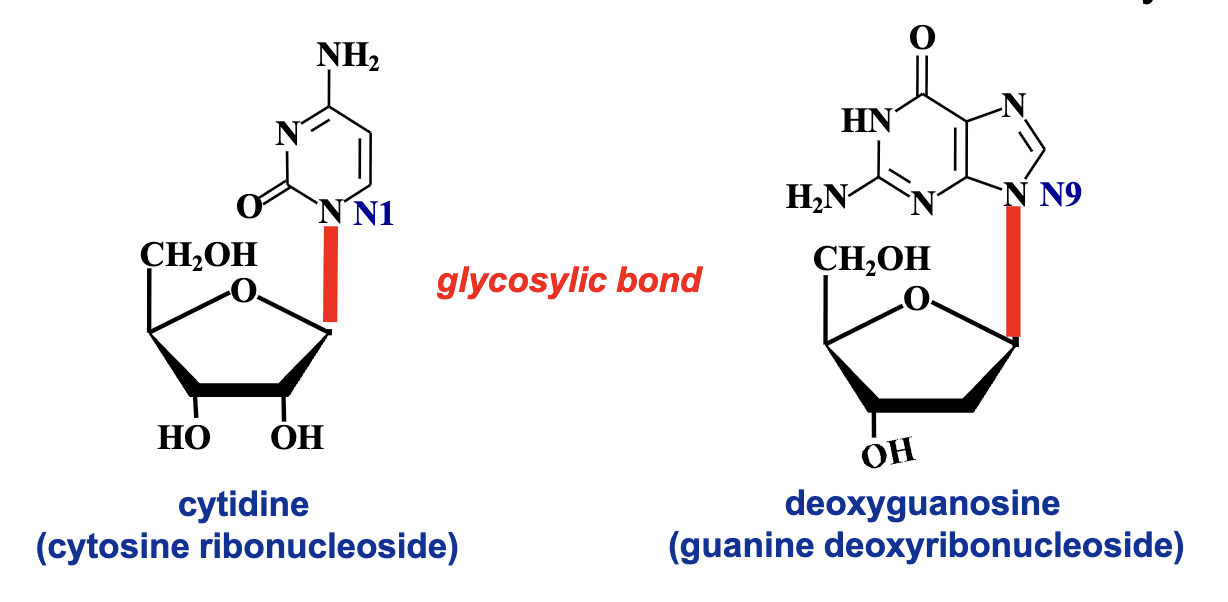
Which nitrogen atom of purines and pyrimidines links to the sugar?
The NH at position 9 of purines links to the anomeric carbon of the sugar.
The NH at position 1 of pyrimidines links to the anomeric carbon of the sugar.

What is the difference between a glycosidic bond and a glycosylic bond?
Glycosidic bond: C–O bond linking sugars or sugar to an -OH group.
Glycosylic bond: C–N bond linking a sugar to a nitrogenous base (in nucleosides).
Bases and their corresponding nucleosides

so for example when adenine is bound to ribose its called adenosine, but when its bound to deoxyribose its deoxydenosine
anything with deoxy is DNA, anything without oxy is RNA, except thymine where it is not in RNA.
T is starred to mention that there is no d in front but it is assumed to be deoxy.
Nucleotide
A phosphorylated nucleoside, consisting of
a nitrogenous base,
a sugar (ribose or deoxyribose),
one or more phosphate groups.
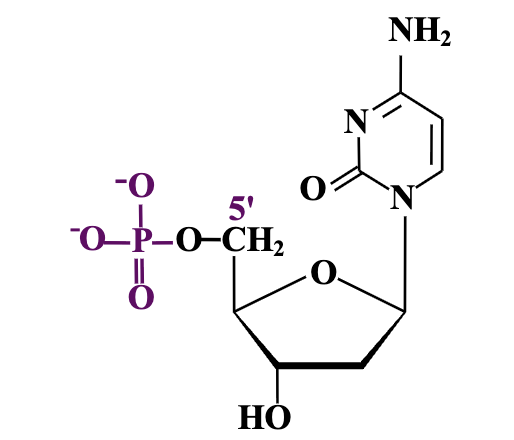
when drawing figures, H atoms are left out for simplicity
How are nucleotides formed from nucleosides?
By adding one or more phosphate groups to the 5′ carbon of the sugar in the nucleoside.
How are nucleotide units linked in DNA and RNA?
Through phosphodiester bonds, which connect the 5′ phosphate group of one nucleotide to the 3′ OH group of the next - so phosphate groups connect them.
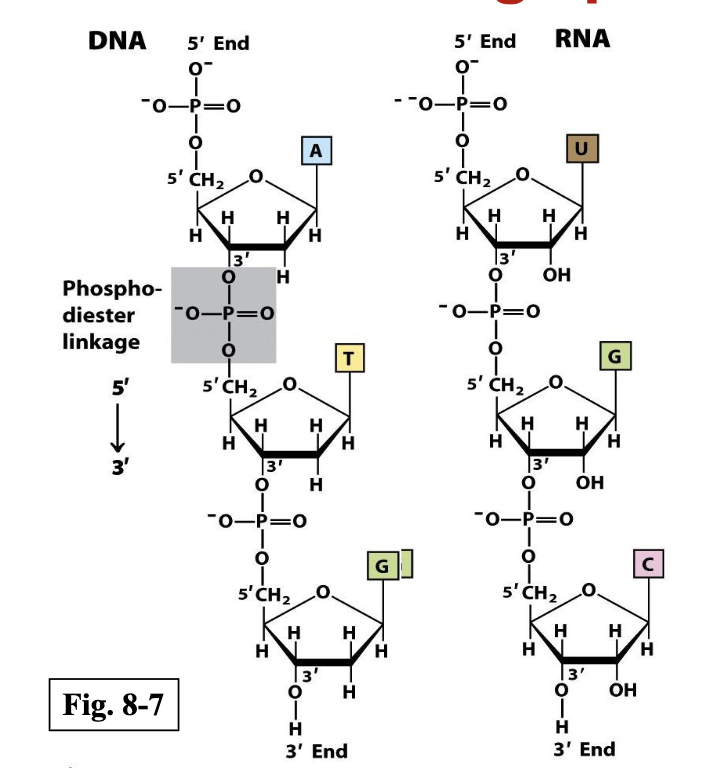
5’-3’ directionality
Are DNA and RNA negatively or postively charged?
negatively, because the phosphate groups in the backbone are completely ionized and negatively charged at physiological pH (~7).
The net charge on the RNA oligonucleotide 5′UGCUAC3′(bearing – OH groups at the 5′and 3′ends, at neutral pH, and ignoring any counter ions) will be
There are 5 phosphate groups in 5′UGCUAC3′ and phosphbate has a charge of -1, so the total charge is -5
if it had one more phosphate group it would be -6
Phosphate groups are what give RNA a negative charge.