Topic 11: Corrosion
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
the deterioration of the material resulting from chemical attack by its environment
what is corrosion

electro → movement of electrons, chemical → changes in chemical composition
consider sodium chloride added to water:
→ NaCl granules dissolve
→ the sodium and chlorine atoms separate or “dissociate” to form the ion species Na+ and Cl-
→ the positively charged ion (Na+) = cation
→ the negatively charged ion (Cl-) = anion
→ a solution containing dissolved ions is called an electrolyte
describe the electrochenical process of corrosion
reaction by which metals form cations that go into aqueous solution (water based), i.e. reaction by which metal corrodes
called the anodic reaction
the location where this reaction takes place is called the anode
the electrons generated in this reaction remain in the metal
what is an oxidation reaction
reaction where a metal or non-metal cation accepts electrons
called the cathodic reaction
the location where this reaction takes place is called the cathode
this reaction consumes electrons
what is a reduction reaction
at the same time
at the same rate
what time and rate must oxidation and reduction reaction occurs at
2H+ (aq) + 2e- (aq) → H2(g)
what is the reaction for corrosion occurring in acidic solutions (containing H+ ions) and no metal ions
O2(g) + 4H+(aq) + 4e-(aq) → 2H2O(l)
what is the reaction for corrosion occurring in an oxidising acidic solution with no metal ions
O2(g) + 2H2O(l) + 4e- (aq) → 4OH-(aq)
what is the reaction for corrosion occurring in a neutral or alkaline solution with no metal ions
if a piece of iron is left outside it will undergo uniform corrosion and form ‘rust’
in this case, water from rain or condensation, which contains some dissolved oxygen from the air, sits on the surface
the anodic corrosion reaction that occurs at microscopic local anodes is:
→ 2Fe(s) → 2Fe2+ (aq) + 4e-the cathodic reaction that occurs at a microscopic local cathode is:
→ O2(g) + H2O(l) + 4e- (aq) → 4OH- (aq)the overall reaction in this case is the sum of these:
→ 2Fe(s) + O2(g) + 2H2O(l) → 2Fe2+(aq) + 4OH-(aq)if the surface is allowed to dry, the ferrous hydroxide dehydrates to form ferric oxide, Fe2O3:
→ 2Fe(OH)2 (aq) → Fe2O3 . 3H2O — rust
describe the rusting of iron
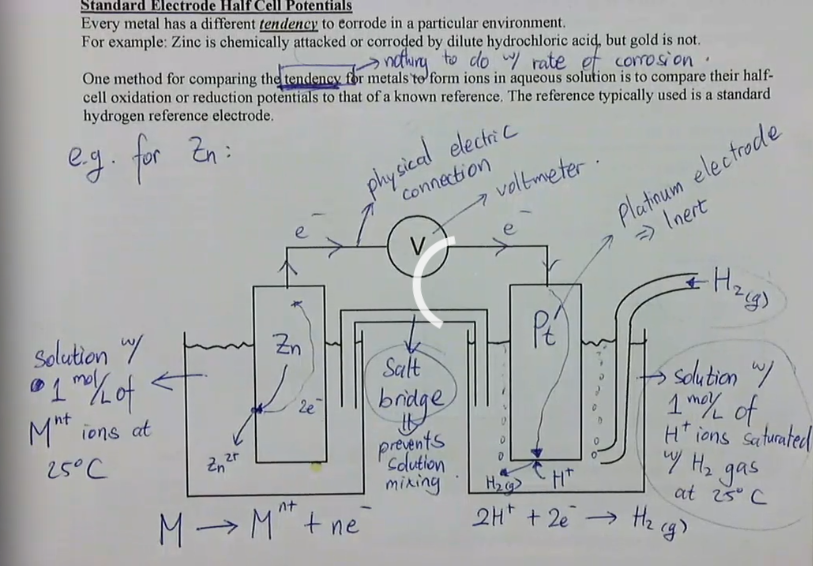
Every metal has a different tendency to corrode in a particular environment.
For example: Zinc is chemically attacked or corroded by dilute hydrochloric acid, but gold is not.
One method for comparing the tendency for metals to form ions in aqueous solution is to compare their halfcell oxidation or reduction potentials to that of a known reference. The reference typically used is a standard hydrogen reference electrode.
Platinum is the standard hydrogen reference electrode
draw the electrodes used in electrolysis
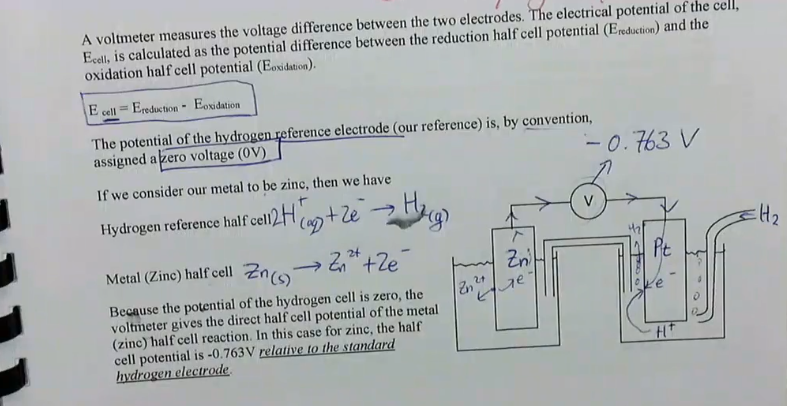
Ecell = Ereduction - Eoxidation
the potential of the hydrogen reference electrode is by convention assigned a zero voltage (0V
metals which are more reactive than hydrogen are assigned negative potentials
if we connect the metal to the hydrogen reference electrode without a voltmeter, these metals are oxidised to ions and hydrogen ions are reduced to form hydrogen gas
the reaction with the more negative electropotential → will be oxidised
the reaction with the most positive electrode potential → will be reduced
how is the half-cell potential calculated

the galvanic series represents the relative reactivities of the listed metals and alloys
metals/alloys near the top are cathodic/noble/inert/unreactive
i.e. there is a decreasing tendency to corrode in moving up the table
metals/alloys near the bottom are increasingly anodic/reactive
i.e. there is an increasing tendency to corrode in moving down the table
no voltages are provided, but the table works in the same way as for the standard electrode potentials — materials lower down the table have the greater tendency to corrode
this galvanic series differs from the standard electro potential table in that it includes common alloys as well as elemental metals
some of the alloys are listed twice, in both active and passive conditions
active: conditions where alloy cannot form a stable oxide layer
passive: conditions where alloy can form a stable oxide layer
the presence of a stable oxygen containing film (oxide layer) on the surface shifts these alloys towards the cathodic (less reactive) end of the series
galvanic series
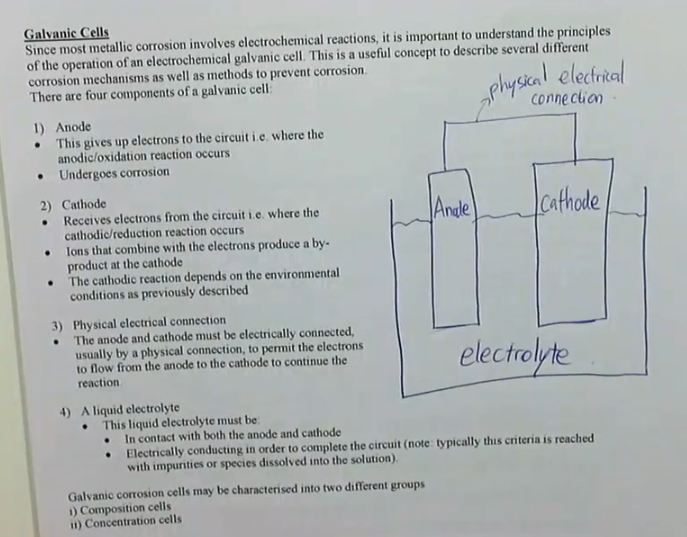
since most metallic corrosion involves electrochemical reactions, it is importat to under the principles of the operation of an electrochemical galvanic cell, this is a useful concept to describe several different corrosion mechanics as well as methods to prevent corrosion
there are four components of a galvanic cell
1. anode
this gives up electrons to the circuit, i.e. where the anodic/oxidation reaction occurs
undergoes corrosion
2. cathode
receives electrons from the circuit, i.e. where the cathode/reduction reaction occurs
ions that combine with the electrons produce a by-product at the cathode
the cathodic reaction depends on the environmental conditions as previously described
3. physical electrical connection
the anode and cathode must be electrically connected, usually by a physical connection to permit the electrons to flow from the anode to the cathode to continue the reaction
4. a liquid electrolyte
this liquid electrolyte must be:
in contact with both the anode and cathode
electrically conducting in order to complete the circuit (note: typically this criteria is reached with impurities or species dissolved into the solution)
galvanic corrosion cells may be characterised into two different groups:
composition cells
concentration cells
galvanic cells
a composition cell may be established between and two dissimilar metals
in each case the metal lower on the standard electrode potential (or galvanic series) table acts as the anode and undergoes corrosion
the further apart the two metals are, the greater the potential/tendency for corrosion to occur
during corrosion the metal atoms at the anode go into solution and release electrons
the cathode is protected from corrosion in this process
typically, if either metal were exposed to the electrolyte by itself, it would corrode, but at a comparatively slow rate — they would undergo general corrosion
the danger comes when they are coupled together i a galvanic cell — the corrosion rate of the anode metal in this case is much greater than its rate of general corrosion
in addition, the cathode material is protected from undergoing corrosion by this process
what are composition cells
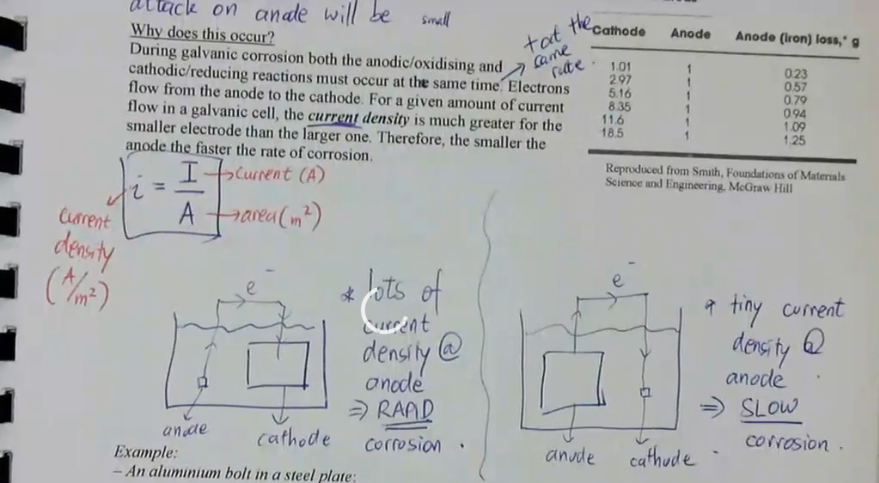
the other factor that controls galvanic corrosion is the relative size of the anode and cathode
if the anode is small relative to the cathode:
attack on anode will be large
if the anode is large relative to the cathode:
attack on anode will be small
why does this occur:
during galvanic corrosion both the anodic/oxidising and cathodic/reducing reactions must occur at the same time and at the same rate
electrons flow from the anode to the cathode
for a given amount of current flow in a galvanic cell, the current density is much greater for the smaller electrode than the larger one
therefore, the smaller the anode, the faster the rate of corrosion
current density equation: i = I/A (current/area), measured in A/m²
how does area effect corrosion
the amount of metal removed from the anode by corrosion can be determined by Faraday’s equation
m = (I x t x M)/(n x F)
m = mass in grams of metal lost
I = corrosion current in amps
t = time in seconds
M = atomic mass of the metal (g/mol)
n = number of electrons exchanged in the anodic reaction
F = Faraday's number (96,500 C/mol or amp.sec/mol)
what is the rate of metal loss to corrosion (for uniform corrosion)
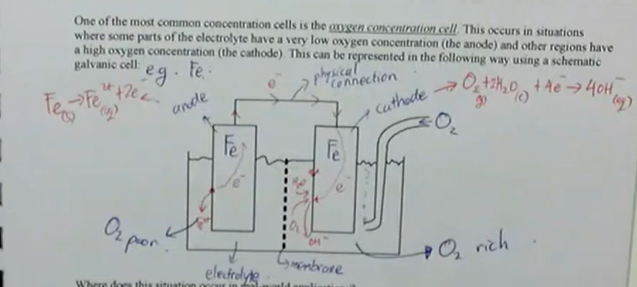
concentration cells develop due to differences in the concentration of the electrolyte, in this situation:
the metal in contact with the most concentrated solution will be the cathode (protected)
the metal in contact with the dilute solution will be the anode (will corrode)
one of the most common concentration cells is the oxygen concentration cell, this occurs in situations where some parts of the electrolyte have a very low oxygen concentration (the anode) and other regions have a high oxygen concentration (the cathode) — this can be represented in the follow way using a schematic galvanic cell
concentration cells
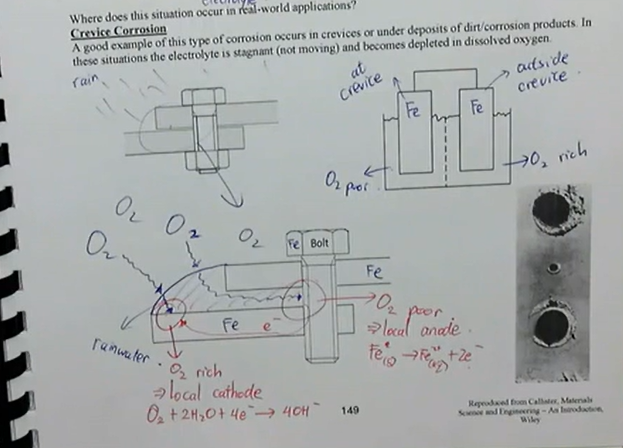
crevice corrosion is an example of a concentration cell
occurs in crevices or under deposits of dirt/corrosion products, in these situations the electrolyte is stagnant (not moving) and becomes depleted in dissolved oxygen
what is crevice corrosion

pitting corrosion is another form of localised corrosion attack where small pits or holes form by the same mechanism as for crevice corrosion
once formed, it develops vertically downwards
important in alloys that rely on an oxide layer for protection
once pits form, the low oxygen concentration at the bottom makes it difficult for an oxygen layer to form, i.e. no protection from corrosion
what is pitting corrosion
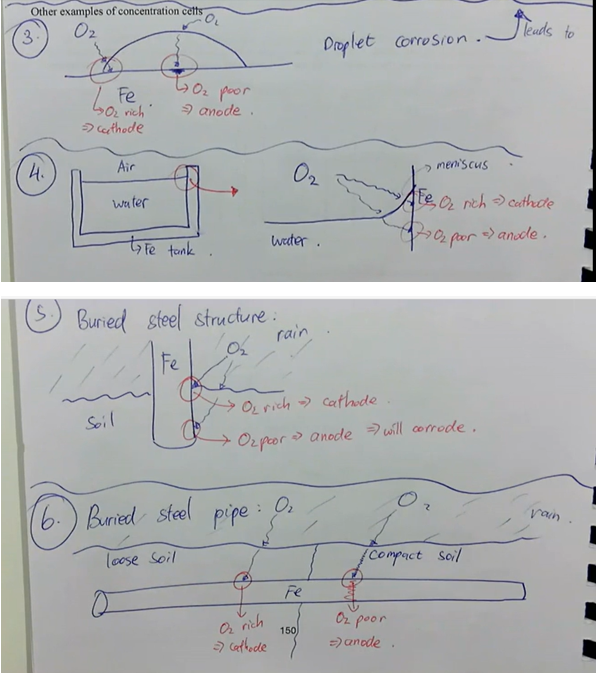
droplet corrosion → leads to pitting corrosion
water line corrosion
buried steel structure
buried steel pipe
what are other examples of concentration cells/corrosion
remove the electrolyte
design components so water does not sit for extended periods
clean surface deposits which may trap moisture near the surface
stagnant water forms O2 concentration cells
change materials so that the anodic material becomes cathodic
use of galvanising and sacrificial anodes
e.g. Zn-coating on steel
cathodic protection
cover the cathode to isolate it from the system
apply protective coatings
e.g. paint
passive layer (e.g. stainless steel)
electroplating (e.g. Sn-coated steel)
remove the electrical connection between the anode and cathode
use insulating materials between dissimilar materials
how do we prevent corrosion
corrosion of a metal, M, occurs by the generalised reaction
M(s) → Mn+ (aq) + ne-
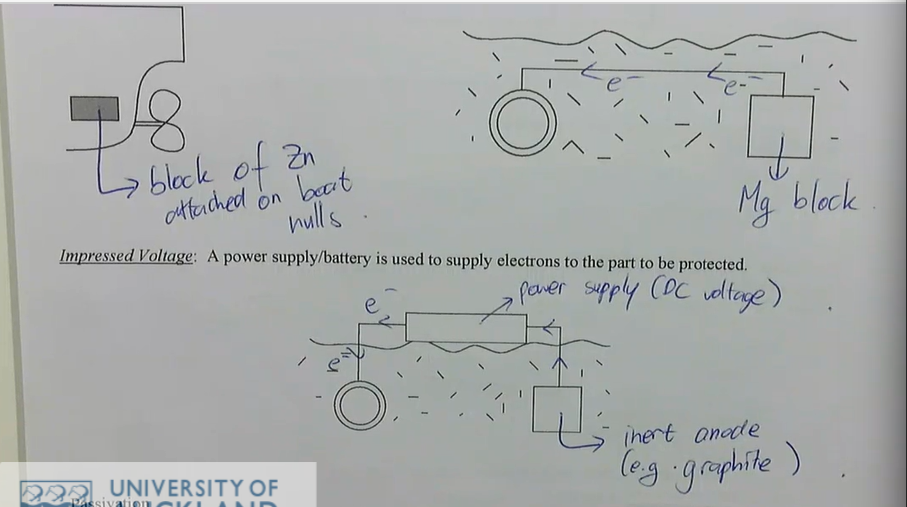
cathodic protection works by continuously supplying electrons to the metal to be protected, forcing it to be the cathode
there are two methods of supplying electrons to the metal:
sacrificial anode: a metal that is more reactive on the galvanic series is attached to the metal to be protected and acts as a sacrificial anode
as it corrodes it provides electrons to the metal to be protected
it must eventually be replaced
typically use magnesium or zinc as sacrificial anodes
examples: protection of buried pipes, ships, offshore drilling platform
impressed voltage: a power supply/battery is used to supply electrons to the part to be protected
cathodic protection