MCAT Biochemistry
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Enzyme functions (4)
provide suitable environment for reaction, provide amino acid residues to act as acids/bases, lover activation energy of transition state, weaken bonds of reactants
Saturated hydrocarbon
A carbon molecule that only has single bonds to hydrogen
Urea cycle
process that occurs in the liver that removes excess nitrogen from the body through amino groups like ammonia
What does D and L glucose look like
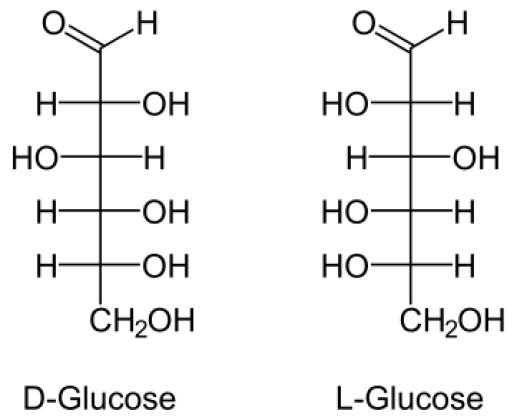
What is a epimer
A configuration of a molecule that is different at only one chiral carbon
What is the difference between a alpha and beta glyosidic bond
Ass DOWN, Boobs UP
What is the pKa of the carbonyl group and when is is de/protonated
2 and its protonated <2 and deprotonated >2 (-)
What is the pKa of the amino group and when is it de/protonated
9 and its protonated <9 (+) and deprotonated >9
What does the Gq protein do (describe pathway)
Gq activates phospholipase C, which cleaves the membrane forming PIP2. PIP2 is cleaved into DAG and IP3. IP3 can open calcium channel in the ER with increases intracellular Ca
What is amphipathic mean?
Having both polar and nonpolar regions in one molecule
What is the charge of the DNA backbone
The backbone of DNA has a negative charge due to the phosphates
What does pyruvate dehydrogenase do
Turns pyruvate to acetyl CoA which will eventually lead to ATP production
Contrast the glucose production of the liver and muscles
Liver creates glucose to balance body wide glucose levels while muscle creates glucose to use for itself
In an isoelectric focusing experiment, explain where a negative and positive protein would move in it
In isoelectric focusing, electrons move from the anode to the cathode (electric field). A negative protein will not move with the electric and move toward the anode. A positive protein will move with the electric field toward the cathode.
Compare SDS PAGE and reductive SDS PAGE
SDS PAGE breaks non-covalent bonds while reductive SDS PAGE breaks down covalent bonds (like disulfide bridges)
Proteases are ____ and only cleave __-amino acids
Proteases are stereospecific and only cleave L-aminoacids
In a enzyme activity plot, what does a sigmoidal curve show
Cooperativity
Cofactor
non-protein, inorganic molecule/metal ion that allows enzymes to be effective
Coenzymes/vitamins
small organic molecules that allow enzymes to be effective
Number of Hydrogen bonds between adenine and thymine
2
Number of hydrogen bonds between guanine and cytosine
3
Energy change when breaking a bond
Endothermic
Energy change when forming a bond
Exothermic
What is the structure of a GCPR
A long polypeptide that folds into 7 segments that are imbedded in the membrane
What is a assumption of michaelis-menten kinetics
there is a single binding site for substrate
How does a competitive inhibitor affect Km and Vmax
Increases Km and does nothing to Vmax
How does a uncompetitive inhibitor affect Km and Vmax
Decreases Km and decreases Vmax
How does a noncompetitive inhibitor affect Km and Vmax
Does nothing to Vmax and decreases Vmax
Nonpolar amino acids
Great Adventures Make Valuing Life In Finite Ways Possible
Polar but neutral amino acids
Stoop To Your Caste Now Quitter
Polar but charged amino acids
Killing Red Haunts Dead- Ethereals-