Chemical Reactivity - Equilibrium
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
LC Explanation
State the CHANGE (concentration/pressure/volume)
Direction of eqn shift
LC Statement (in order to partially oppose the change by…)
Predict Concentration of species
State expected observation (colour darken/fade)
(x)
The concentration of x
Equilibrium constant expression for:
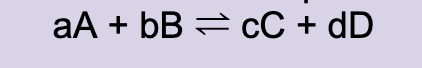
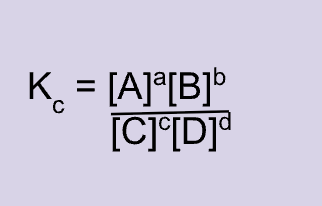
Species to not include in an equilibrium expression
Solids are pure liquids


Favours which reaction?
The forward reaction/ the position of the equilibrium shifts to the right

Favours which reaction?
The reverse reaction/ the position of the equilibrium shifts to the left

Favours which reaction?
The reverse reaction/the position of the equilibrium shifts to the left

Favours which reaction?
The forwards reaction/the position of the equilibrium shifts to the right

Favours which reaction?
The endothermic reaction

Favours which reaction?
The exothermic reaction

Favours which reaction?
The side of the reaction with less gaseous molecules


Favours which reaction?
The side of the reaction with more gaseous molecules
Type 2 LC question
(Change in temp, is the forward reaction endo or exo?)
