Chem 103: Exam 1
1/84
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
85 Terms
When you are multiplying an equation with exponents, you ____ the exponents together
add
When you are dividing an equation including exponents you ____ the exponents
subtract
1m = ____ ft
3 ft
1 km = _____ mi
0.621 mi
1 kg = _____ lb
2.205 lb
1 Liter = _____ gal
0.264 gal
Tera-
10^12
Giga
10^9
Mega
10^6
Kilo
10³
Deci
10^-1
Centi
10^-2
Mili
10^-3
Micro
10^-6
nano
10^-9
pico
10^-12
1 kg =
1000g
1 inch = ___ cm
2.54 cm
Mass number is
protons and neutrons in the nucleus
The atomic number
the amount of protons
Frequency (v)
number of waves per second
PLank’s Constant
6.63 × 10^-34 Js
Speed of Light
3.00 × 10^8 m/s
Level 1 holds
2 e
Level 2 holds
8 e
Level 3 holds
18 e
Level 4 holds
32 e
Sublevel: S
1
Sublevel: p
3
Sublevel d:
5
siublevel f:
7
Electron confidgeraitons order
1s², 2s², 2p^6, 3s², 3p^6, 4s², 3d^10, 4p^6, 5s², 4d^10, 5p^6
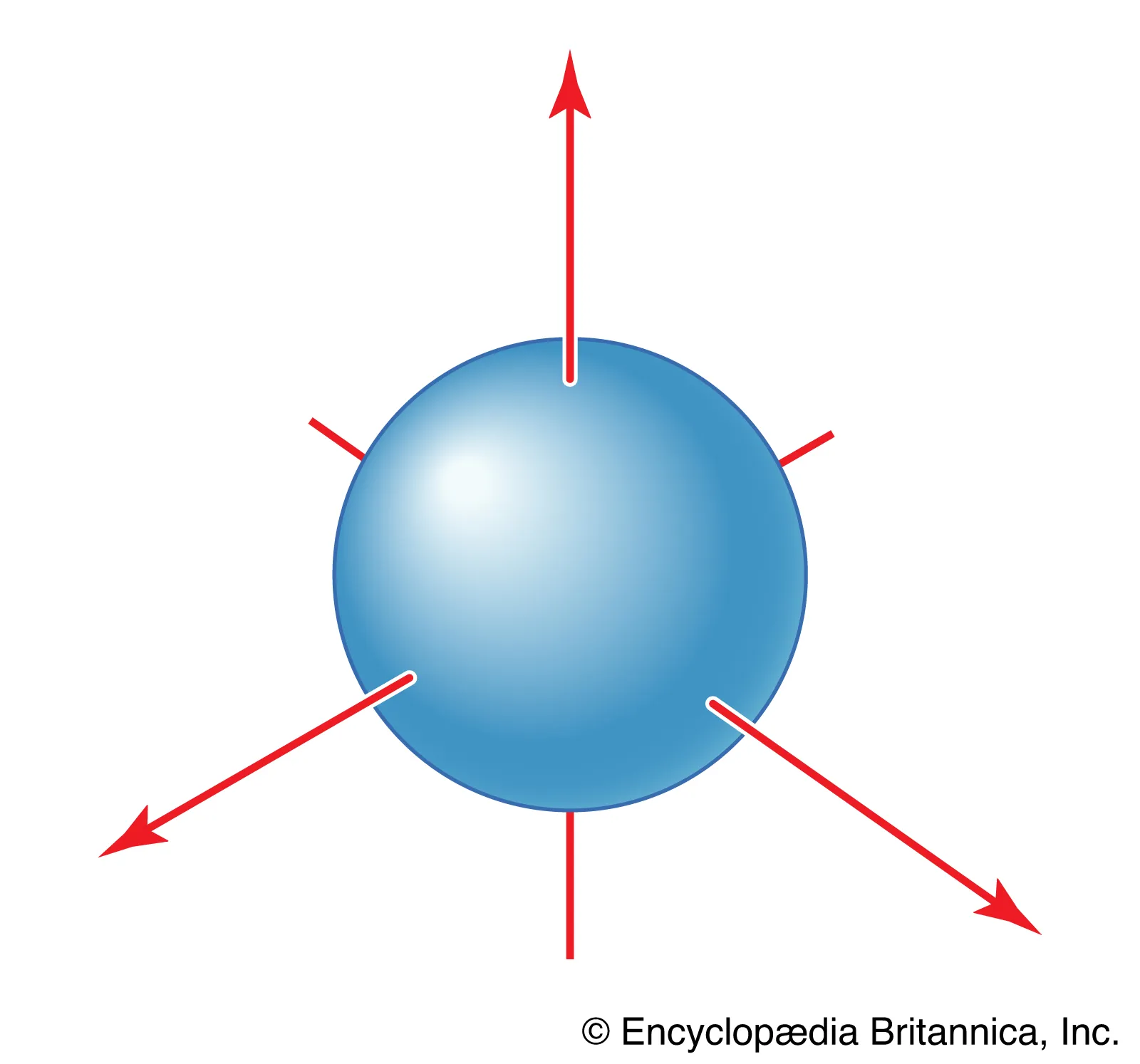
What orbital is this?
S
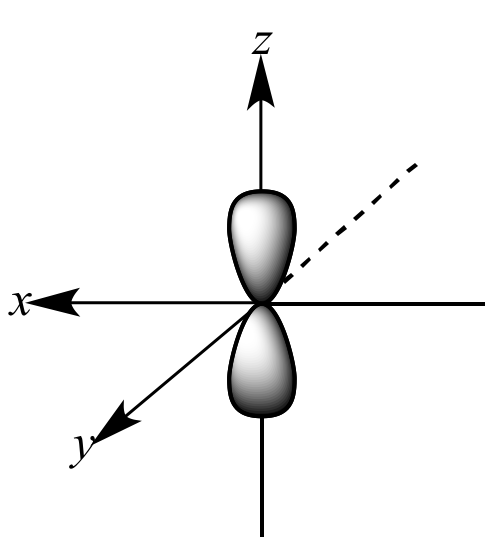
What orbital is this?
P
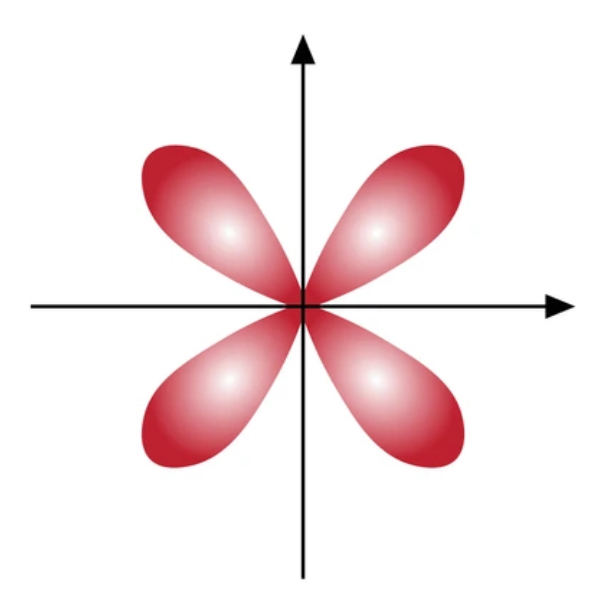
What orbital is this?
D
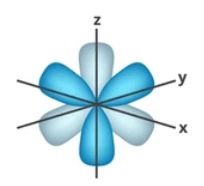
What orbital is this?
f
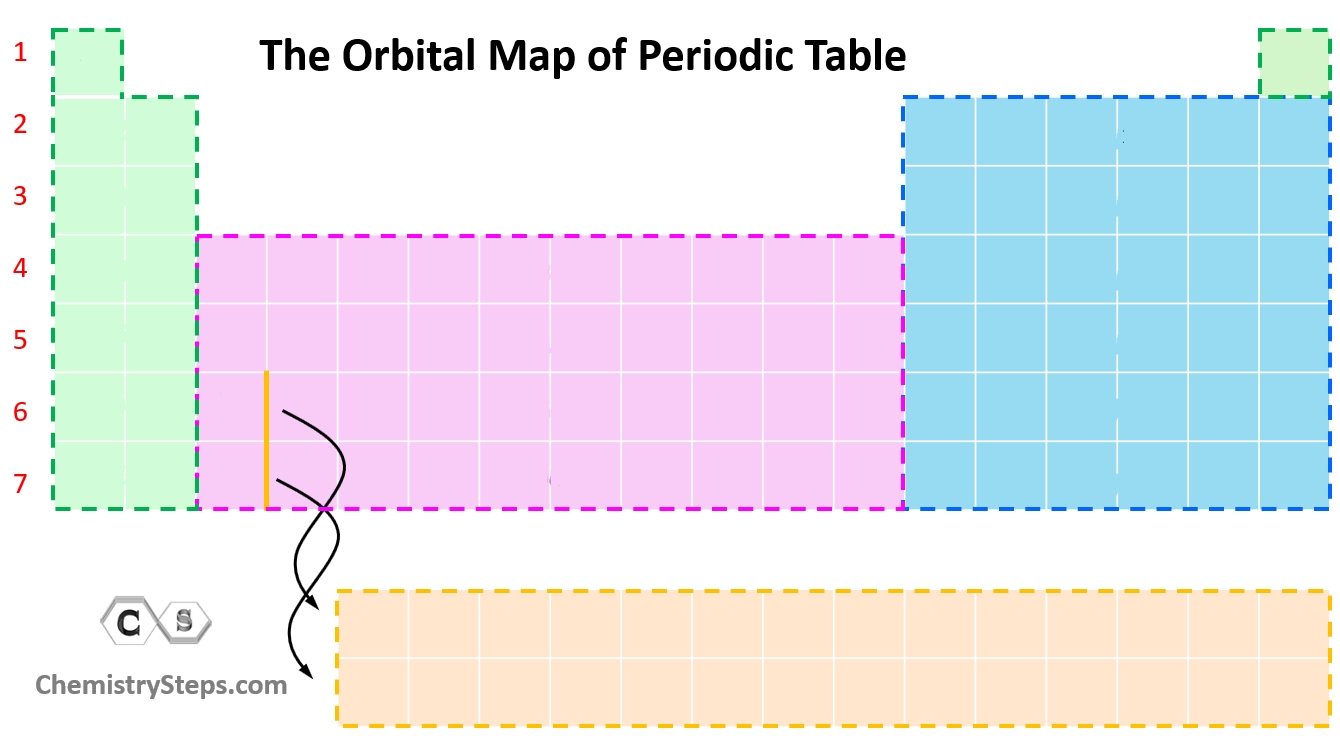
What orbital is the green color?
S
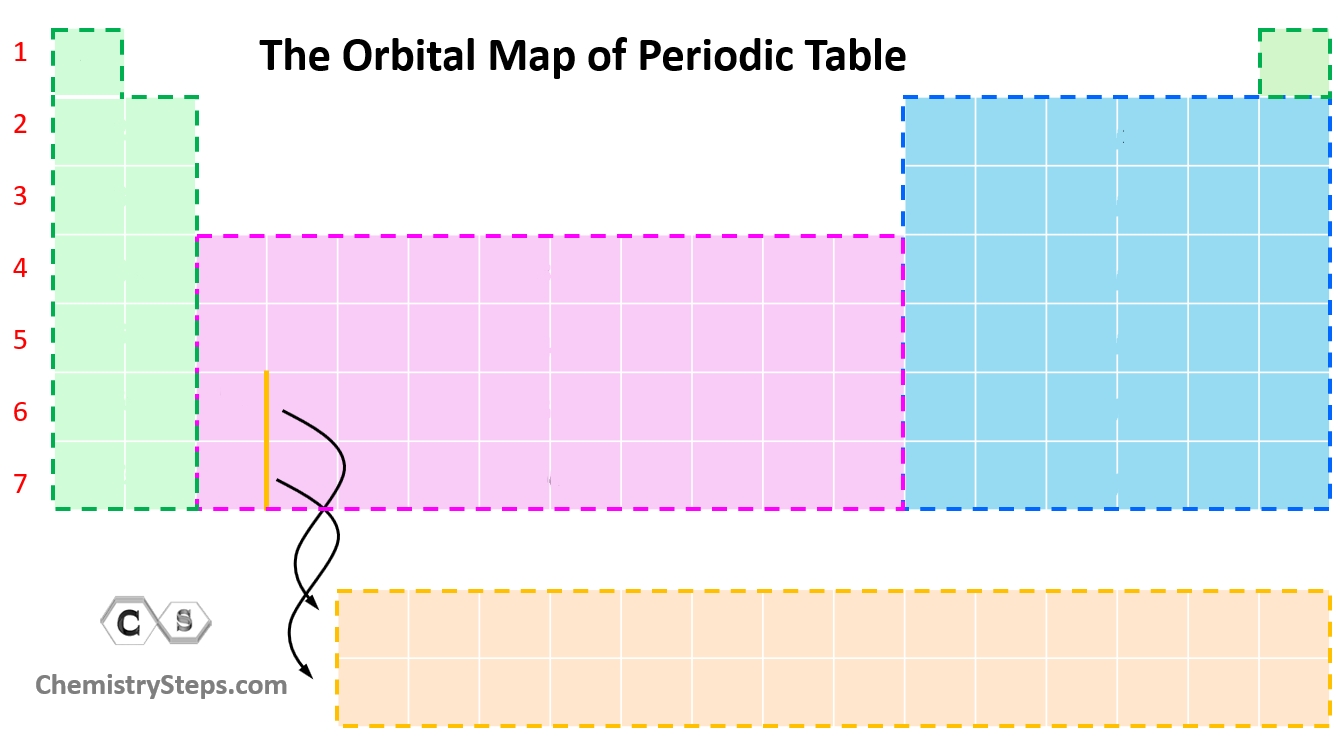
What orbital is the blue color?
p
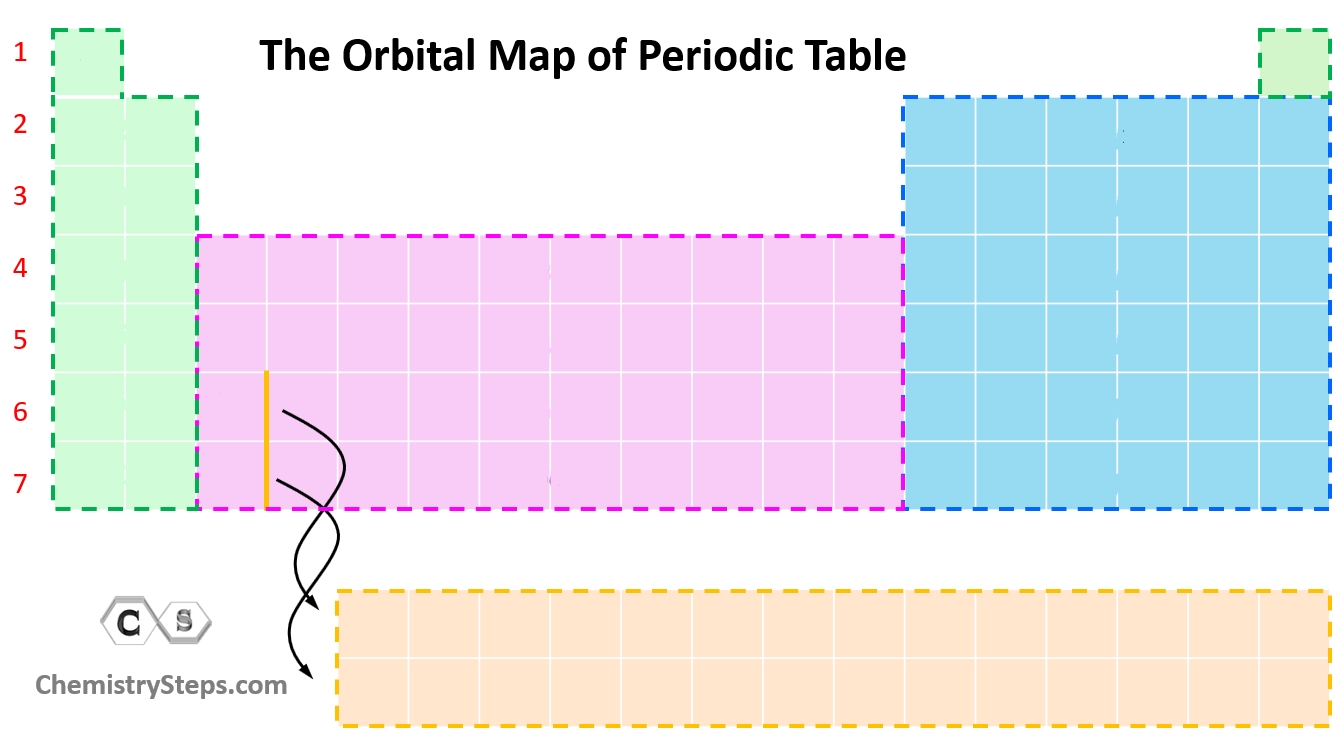
What orbital color is pink?
d
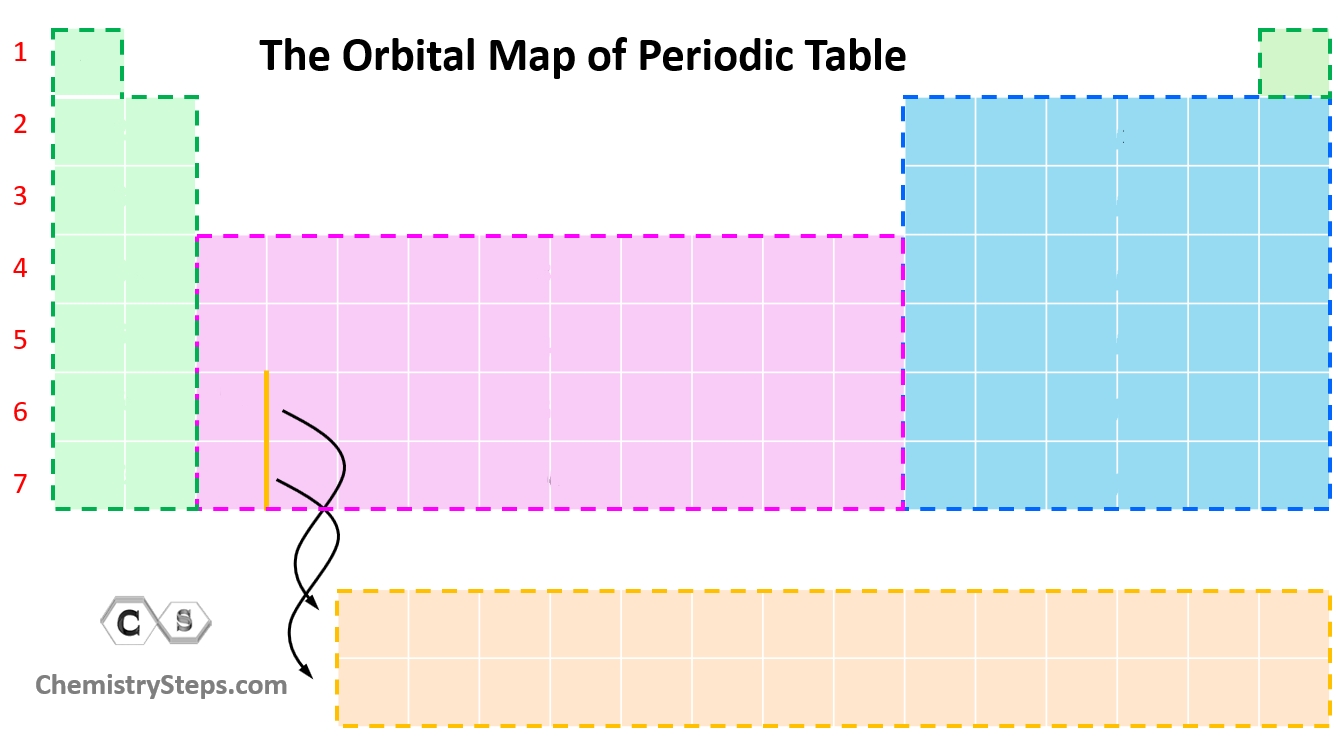
What orbital is the orange?
f
1 Atomic Number
Hydrogen (H)
2 Atomic Number
Helium (He)
3 Atomic Number
Lilthium (Li)
4 Atomic Number
Berryliuum (Be)
5 Atomci Number
Boron (B)
6 Atomic Number
Carbon (C)
7 Atomic Number
Nitrogen (N)
8 Atomic Number
Oxygen (O)
9 Atomic Number
Flourine (F)
10 Atomic Number
Neon (Ne)
Atomic Number 13
Aluminum (Al)
atomic number 11
Sodium (Na)
Atomic Number 12
Magnesium (Mg)
Atomic Number 14
Silicon (14)
Atomic Number 15
Phosphorus (P)
Atomic Number 16
Sulfur (S)
Atomic Number 17
Chlorine (Cl)
Atomic Number 18
Argon (Ar)
Atomic Number 19
K (Potassium)
Atomic Number 20
Calcium (Ca)
Halogens
Flourine (F), Chlorine (Cl), Bromine (Br), Iodine (I)
The number of waves passed in a second
Frequency
The lengths of the wave itself
Wavelength
Energy increases when frequency ____
increases
Energy increases when wavelength
decreases
Speed of Light ( c ) =
Wavelength/Frequency (v)
Frequency (v) =
Speed of light ( c ) / Wavelength
Solving for Energy when given frequency
Energy (E) =2.718 Planck’s Constant (h) x Frequency (v)
Solving for Energy when given Wavelength
Energy (E) = Planck’s Constant (h) x Speed of Light ( c ) / Wavelegnth
Frequency Unit
Hz
Wavelength Unit
Nanometers (Nm)
C → K
K = C + 273.15
K → C
C = K - 273.15
F → C
C = 5/9 (F-32)
C → F
F = (C x 9/5) + 32
F → K
K = (F-32)(5/9) + 273.15

Is this accurate and/or precise
accurate and precise

Is this accurate and/or precise
Accurate but imprecise

Is this accurate and/or precise
Inaccurate but precise

Is this accurate and/or precise
Inaccurate and imprecise
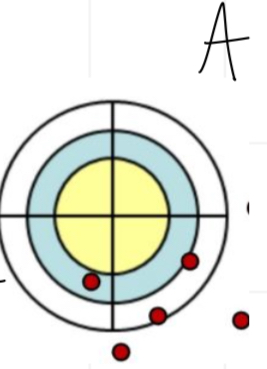
Is this accurate and/or precise
Inaccurate and precise
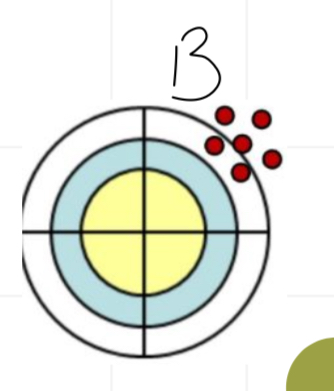
Is this accurate and/or precise
Accurate but not precise
Is this accurate and/or precise
Accurate and Precise
K → F
F = (K-275.15)(9/5) + 32