3.1.3 bonding,forces,bond polarity
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
IONIC BONDS
Explain how an ionic bond is formed? (E.g. sodium and chlorine to make sodium chloride)
Sodium is a metal that has 1 electron in its outer shell and chlorine is a non-metal that has 7 electrons on its outer shell.
Both elements need full outer shells in order to become stable and so sodium will transfer its outer shell electron to chlorine so that they both have full outer shells.
Since sodium has lost an electron it will now have a positive charge and since chlorine gained an electron it will now have a negative charge.
These two elements now have opposite charges and strong electrostatic forces will cause them to join together which we call an ionic bond and the structure of the ions formed will join up into giant lattices .
Name 3 properties of ionic substances?
1.High melting point(many strong electrostatic forces + giant ionic crystal lattice)
2. High boiling point(many strong electrostatic forces + giant ionic crystal lattice)
3. Conducts electricity when aqueous or molten since ions are free to move and so they can carry charge.
What formula compound will be formed from Ga*3+ and SO4*2-?
Ga2(SO4)3
This is because Ga has a 3+ charge and SO4 has a 2- charge. In order to balance the charges you need to times Ga by 2 and SO4 by 3 to make a 6+ charge for Ga and a 6- charge for SO4. Therefore all u need to do is add another Ga atom and times SO4 by 3.
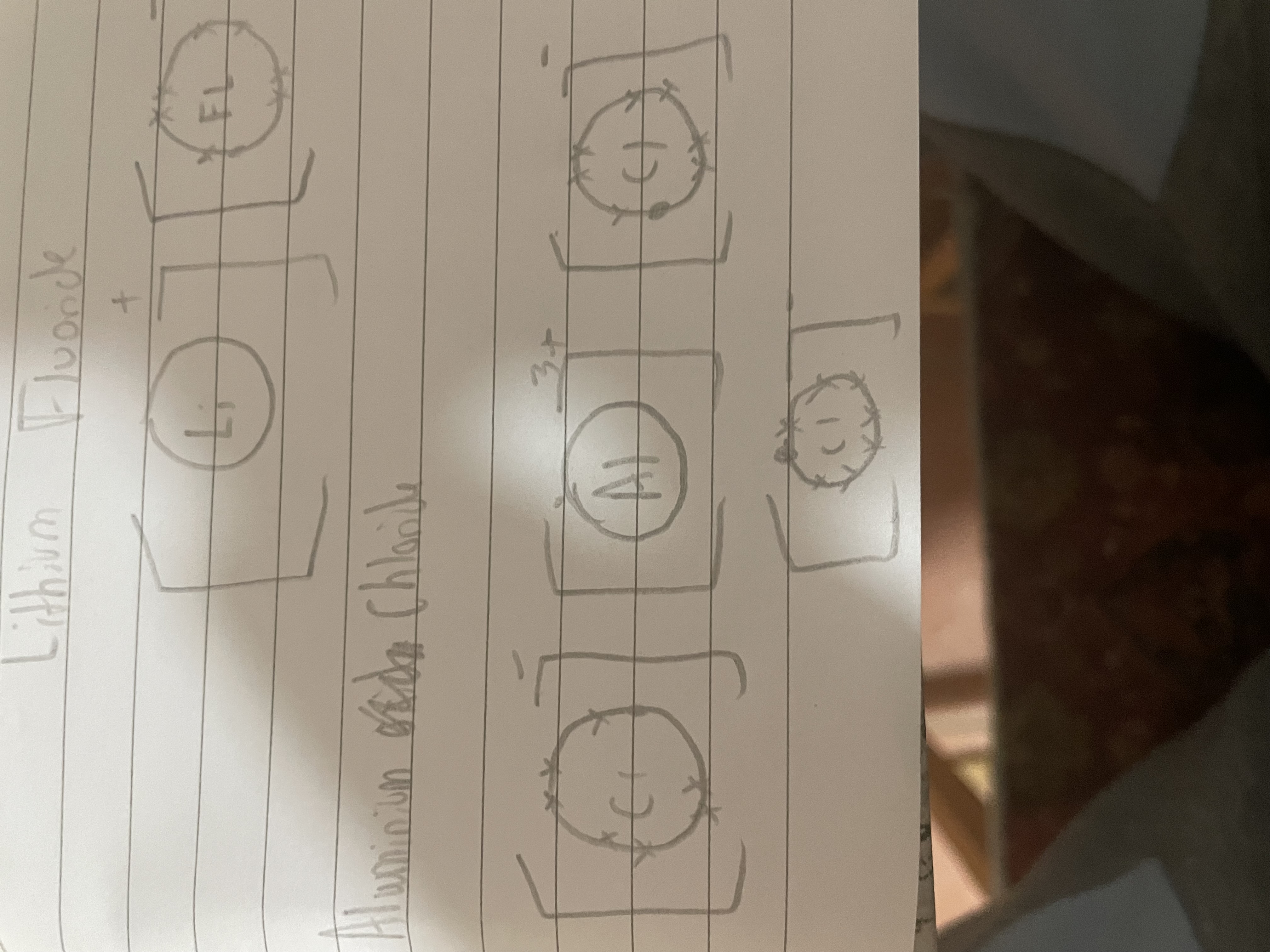
Draw a dot and cross diagram for aluminium chloride (Chlorine has 7 outershell electrons and aluminium has 3 outershell electrons)
COVALENT BONDS
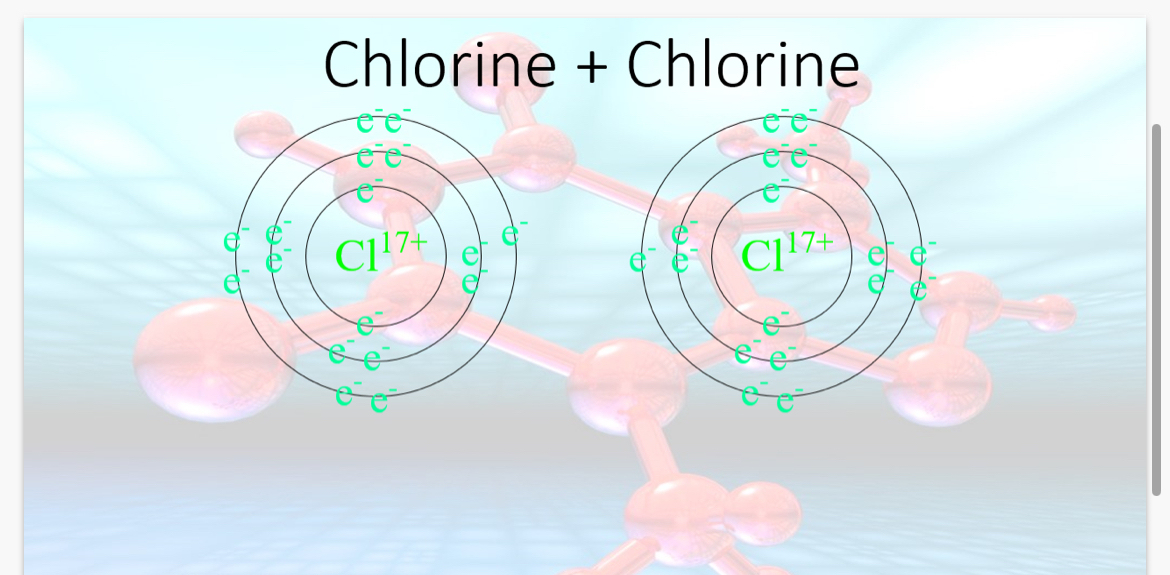
What would a covalent bond between 2 chlorines look like?
How does a covalent bond between 2 chlorine atoms occur?
Unlike your giant ionic crystal lattices these are discrete tiny molecules containing only two atoms. Whilst the bonding between the chlorine atoms is very _1? (not easily broken), the attraction between the molecules is very _2?
Covalent substances with small molecules are often gases or liquids (low Melting point).
The chlorine atoms have 7 electrons on their outer shell and need 1 more electron in order to become stable. This will result in the chlorine atoms sharing their one of their outer electrons in order to gain a full shell.
1.Strong. 2.weak
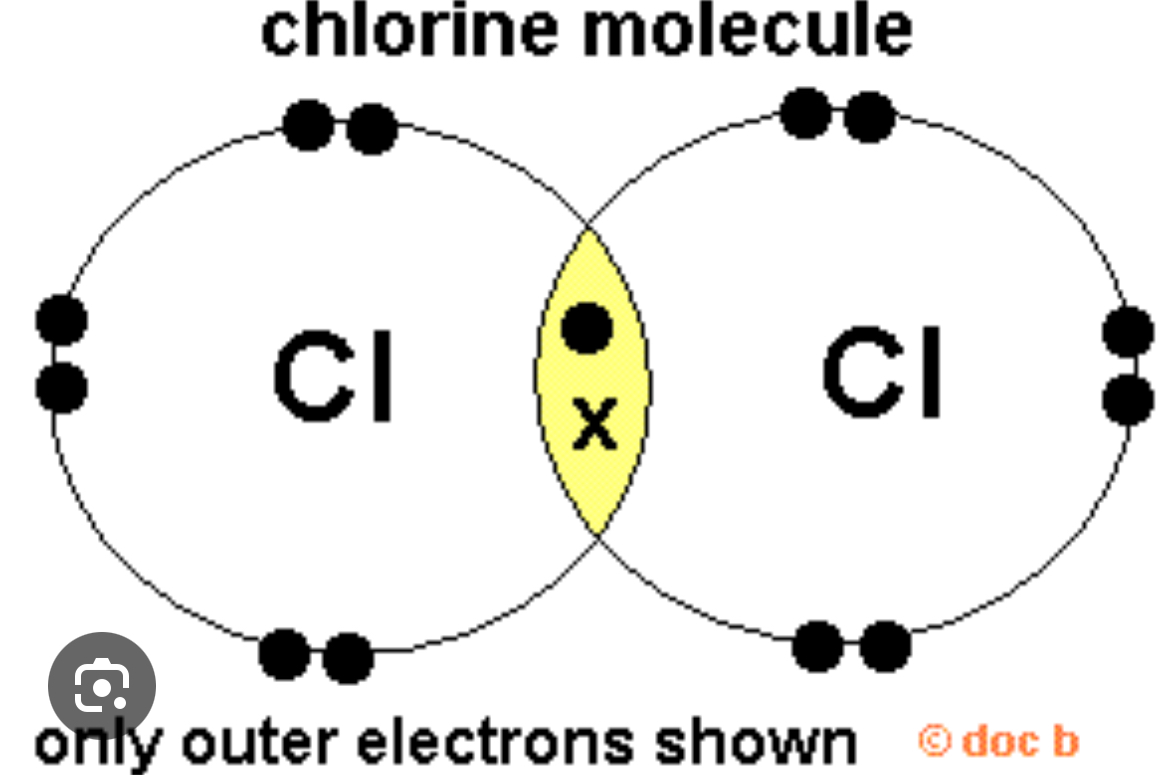
Describe Van der Waal’s forces in chlorine?
Info:The larger a covalent molecule,the greater the Van der waal’s because there are more electrons.
1.Electrons move randomly in the covalent bond.
2.When there is a difference of the number of electrons from one side to the other, one side will become slightly positive and the other will be slightly negative.
3.This creates a temporary dipole in the molecule.
4.The temporary dipole in 1 chlorine molecule induces a dipole in a neighbouring chlorine molecule.
5. The slightly positive end of 1 molecule is then attracted to the slightly negative end of the other molecule which results in a weak electrostatic attraction.
Some covalent compounds have high melting points such as diamond graphite and buckminsterfullerene. Diamond and graphite have very high melting points because of their lattice of strong covalent bonds.
State 5 facts about diamond and graphite
Diamond:•
Each carbon atom is bonded to 4 other carbon atoms in a 3D tetrahedral arrangement,
•No intermolecular forces since it is 1 structure made of many strong covalent bonds
•Extremely hard due to the strong covalent bonds in all directions
•High melting points because a lot of energy is needed to break the strong covalent bonds
•Electrical insulator since there is no delocalised electrons to carry charge.
Graphite:
•A layered structure, each carbon atom is bonded to 3 other carbon atoms in a 2D hexagonal arrangement
•Layers are held together by weak van der waal’s forces.
•High melting point since strong covalent bonds within the layers require a lot of energy to break.
•An electrical conductor since it has delocalised electrons between layers which can carry charge
•Soft and slippery
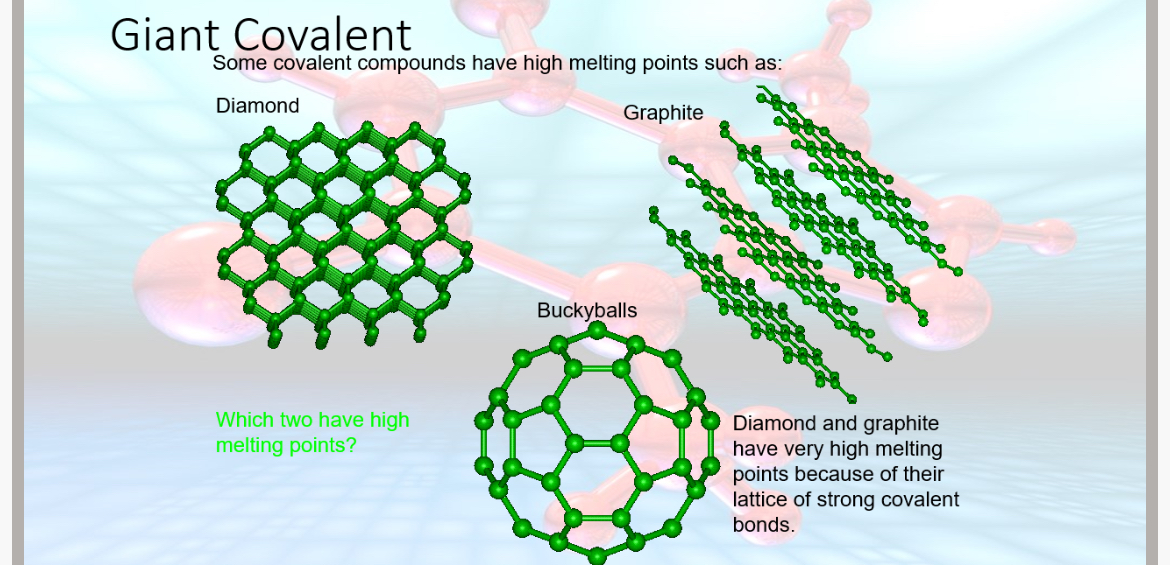
What is the chemical formula for Buckminster fullerene?
C60
METALLIC BONDS
How do metallic bonds form?
* Delocalised Electrons: Metal atoms lose valence electrons, which become free to move throughout the metal structure.
* Positive Metal Ions: Metal atoms become positive ions (cations) and arrange in a lattice.
* Electrostatic Attraction: Delocalised electrons are attracted to the lattice of cations, forming a "sea" of electrons and this holds the lattice structure together.
* Metallic Bond: The overall electrostatic attraction between cations and delocalised electrons holds the metal together.
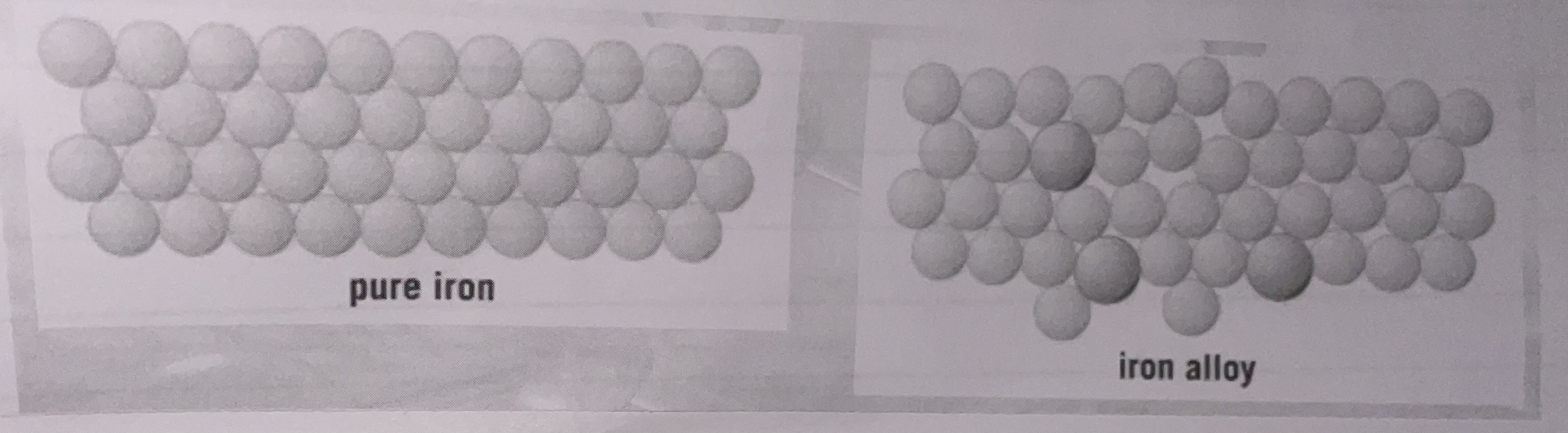
Metals made from only one element are called …1? metals.
Metals made from two or more elements are called …2?.
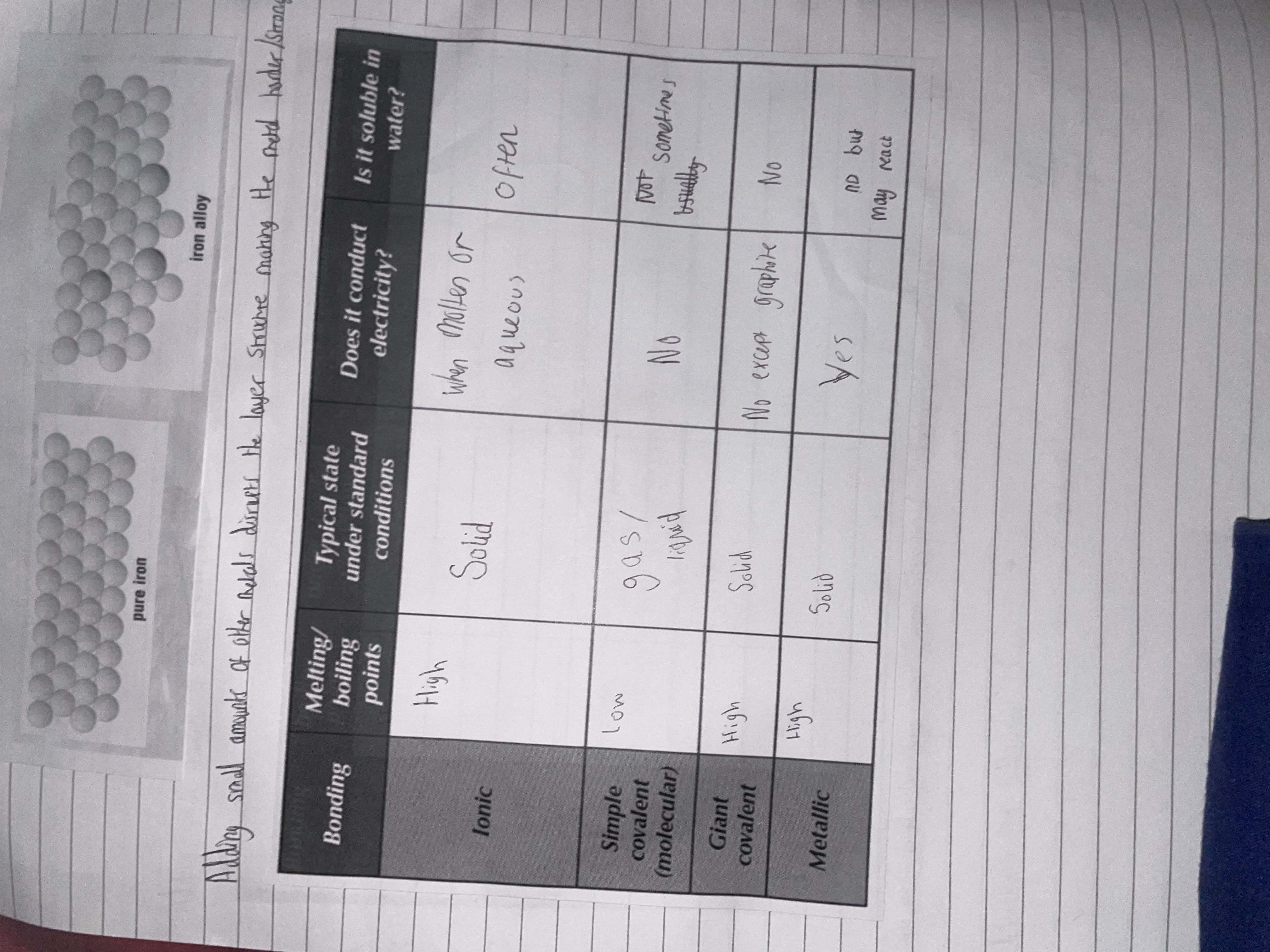
3.Explain why the iron alloy has a different structure to pure iron.
1.pure 2.alloys
3.Adding small amounts of other metals changes the layered structure of iron due to different particle sizes of the other material disrupting iron’s regular lattice structure. (and making it harder for atoms to slide past each-other)
Fill out this table
Why do metals have high boiling and melting points?
there is a strong attraction between cations and delocalised electrons
Why are metals so strong?
Metals have a giant structure and the many electrostatic attractions between metal cations and the delocalised sea of electrons, require a lot of energy to break
1.Why are metals ductile and malleable?
Metals in the liquid state are shiny, still conduct electricity and have metallic bonding but, they are not malleable or ductile because the regular repeating lattice of cations are …2? down when melted which allows for the cations to …3? over each other.This means the metallic bonding is now …4? than when it was in a solid state.
Metals in the gaseous state have …5? of these properties because the metal atoms are too far apart and the electrons become localised and orbit a specific …6? atom.This means there will no longer be …7? or a sea of delocalised electrons.
Metals with stronger metallic bonding have higher …8? And …9? points.
10.Explain why?
1.layers of cations can slide over each other without breaking the metallic bonding
2.broken. 3.flow. 4.weaker. 5.none. 6.metal. 7.cations. 8.melting. 9.boiling
10.Stronger metallic bonding means a stronger attraction between cations and delocalised electrons.
This means more energy is required to weaken and break these attractions, which, in turn, means higher melting and boiling points.
The strength of metallic bonding increases across a …1? on the periodic table.
Across a period the melting points increase because they form …2? with more highly charged cations and they delocalise more …3? per cation which increases the strength of metallic bonding.
Metals dont form more delocalised electrons because metals in groups 1,2 and 3 only form delocalised electrons with the ones in its …4? Shell.
1.period. 2.lattices. 3.electrons. 4.outer
As you go down a group, the strength of metallic bonding …1?.
Why is this the case?
1.decreases
Because the distance between cations and delocalised electrons increases since atoms get bigger as you go down a group.(more shells)
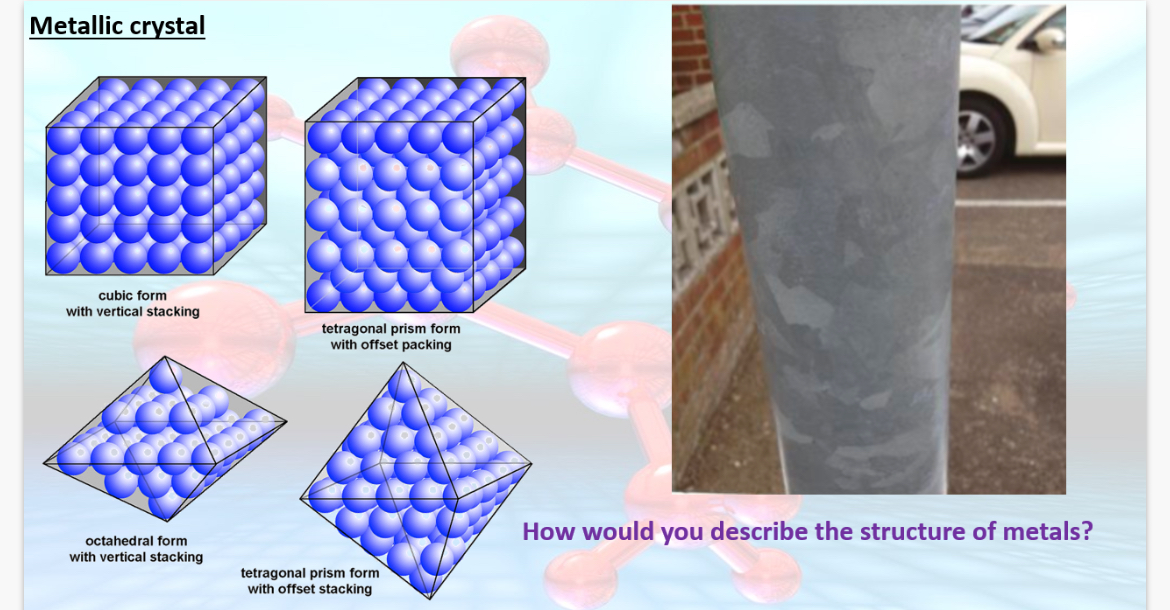
1.Give 3 reasons why aluminium is useful for making cooking pots?
2.What 2 properties of aluminium make it suitable for overhead cables?
3. Name 10 properties of metals?
Note:The image shows metallic crystal structures
1.
•Good conductor of heat due to having delocalised electrons which allow it to carry thermal energy through its structure.
•High melting point-withstand lots of heat
•Strong,Won’t get damaged easily
2.
•Ductile(can be morphed without breaking)
•Conducts electricity
3.High melting point, high boiling point, ductile, malleable(hammer-able),sonorous(ringing noise), heat conductor, electricity conductor, flexible, dense, strong
1.What is equilibrium?
2.What is the octet rule?
3.What is electronegativity?
4.Why is Fluorine the most electronegative element?
1.Reversible reactions where the rate of the forward reaction is equal to the rate of the reverse reaction.(concentrations of reactants and products remain constant)
2.The tendency of atoms to prefer to have 8 electrons in its electron shells(apart from hydrogen and helium)
3.The ability of an atom to pull a bonded pair of electrons in a covalent bond
4.Fluorine has a small atomic radius since it has only 2 shells and this makes it easy to pull in electrons since its positive nucleus is closer to electrons of other elements.
Explain how permanent dipole-dipole attractions occur?
Permanent dipole-dipole attractions happen between polar molecules. Polar molecules have partial positive and negative charges due to unequal sharing of electrons. These molecules align so that the positive end of one attracts the negative end of another. This attraction between permanent dipoles is what we call a permanent dipole-dipole interaction and they don’t require an external force to be induced.
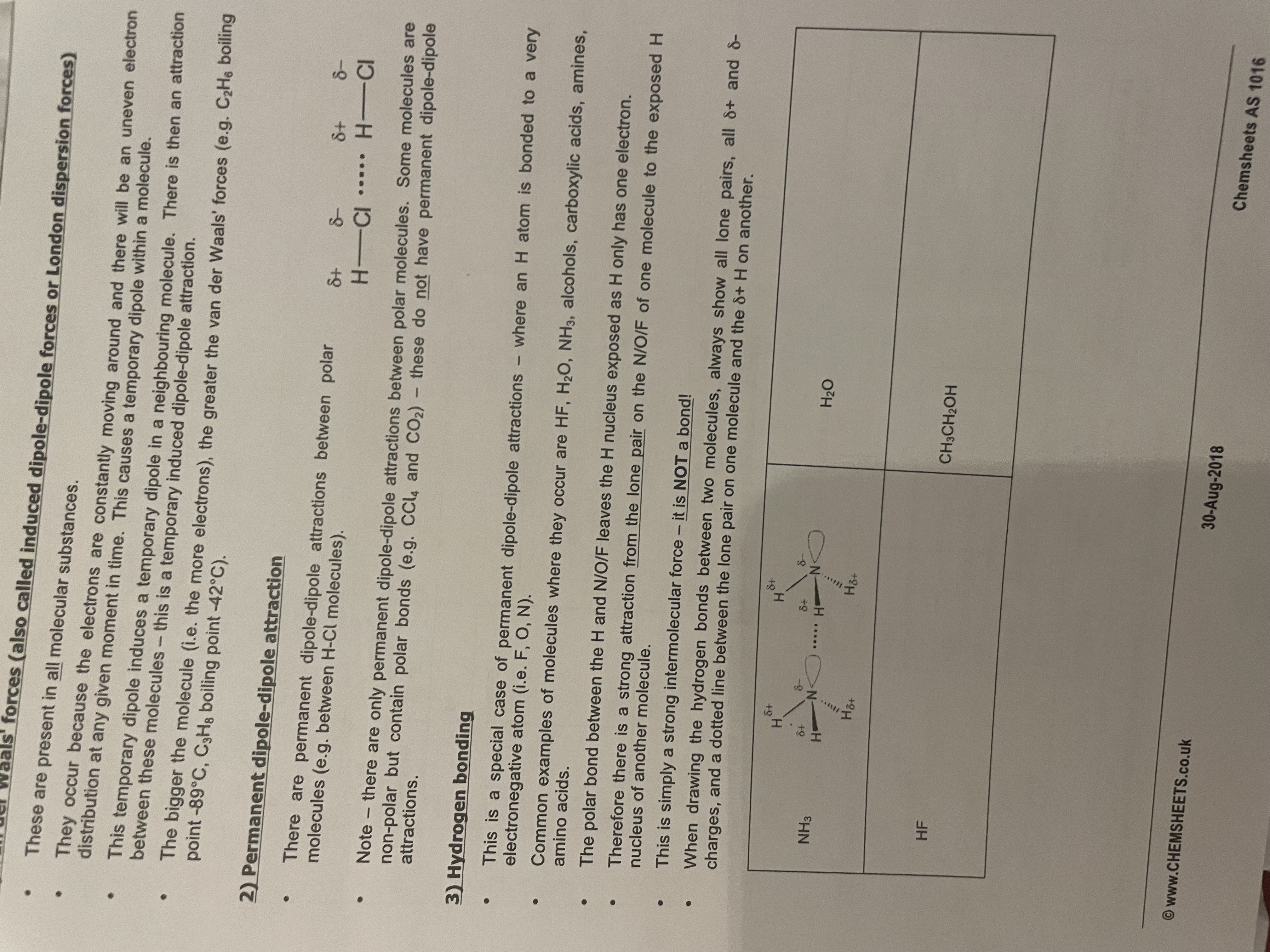
Forces between molecules:
Van der Waal’s forces(aka dipole-dipole or london forces).These are present in …1? molecules.
Permanent dipole-dipole attraction→ These can only be found between …2? molecules e.g. H-Cl molecules. Some non-polar molecules contain polar bonds(e.g. CO2), but they don’t have permanent dipole-dipole attractions.
Hydrogen bonding→ This is a special permanent dipole-dipole attraction where a …3? atom is bonded to a very electronegative atom(i.e. F, O, N)
•common examples of where they occur are HF,H2O,NH3,alcohols,carboxylic acids,amines.
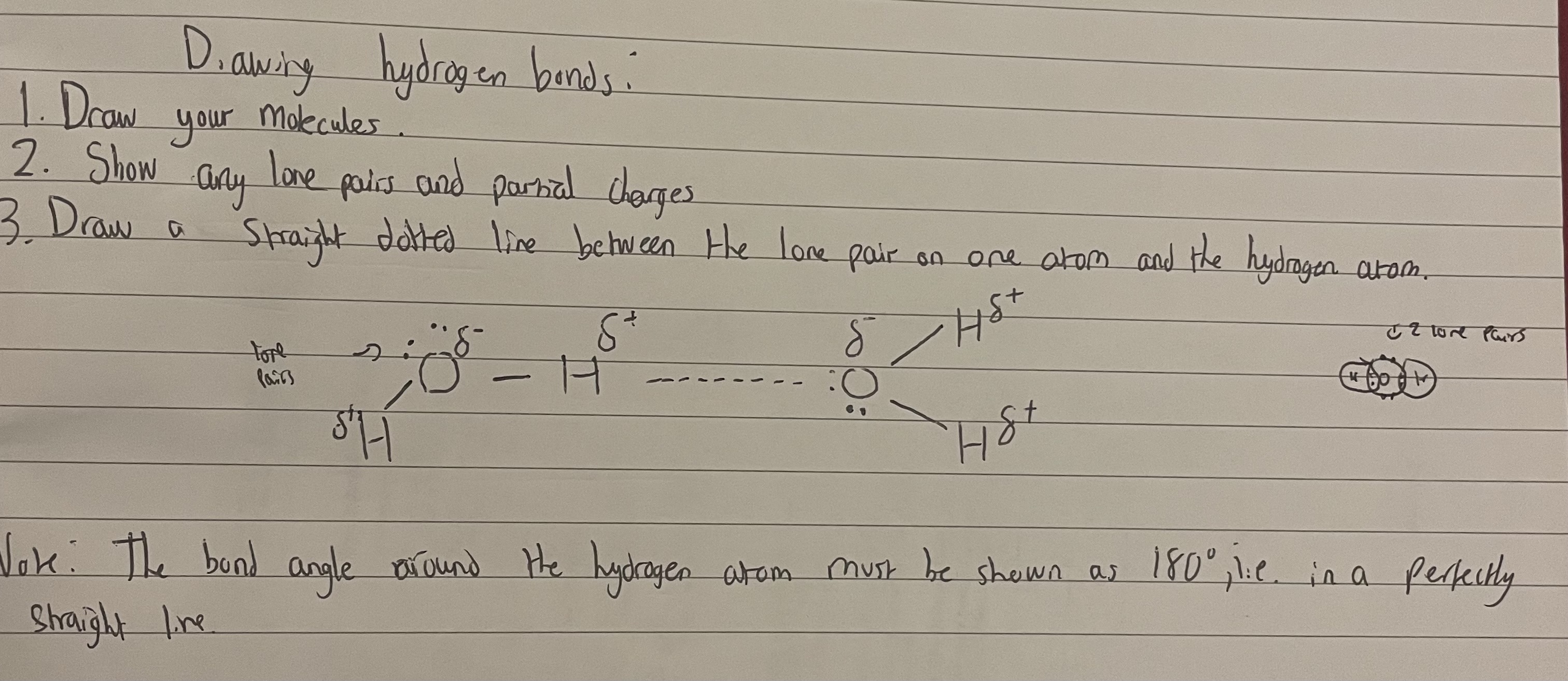
•The polar bond between the H and N/O/F leaves the H nucleus exposed as H only has 1 electron.Therefore, there is a strong attraction from the lone pair on the N/O/F of one molecule to the exposed H nucleus of another molecule.
•This is simply a strong intermolecular force and not a bond.
Note:When drawing the hydrogen bonds between 2 molecules, always show all lone pairs, all delta positive and delta negative charges, and a dotted line between the lone pair one 1 molecule and the delta + H on another.
Note: strength→ VDW’s < permanent dipole-dipole < H-bonding
Note:Molecules can have 1 or 2 of the types of forces but never have all 3.(vdw is present in all)
1.all. 2.polar. 3.H
Explain why iodine has a higher boiling point than fluorine?
Fluorine (F₂) has a lower boiling point than iodine (I₂) because of the differences in the strength of their van der Waals forces.
Iodine molecules are much larger and have more electrons than fluorine molecules. This means that the temporary dipoles in iodine are larger and more easily induced than in fluorine, leading to stronger van der Waals forces. Stronger intermolecular forces require more energy to overcome, resulting in a higher boiling point.
What is the definition of a permanent dipole?
When there is a small charge difference across a bond resulting from a difference in the electronegativities of the bonded atoms.
Name 3 factors that affect electronegativity?
What is the trend in electronegativity when you go down a group and when you go across a period?
1.Number of protons-More protons makes electrons more attracted to the nucleus.
2.Atomic radius-If there is a larger distance from the atomic nucleus to the outer electrons,there will be less attraction.
3.Shielding-A larger number of shells makes it harder to attract electrons since outer electrons will face repulsion from inner electrons which affects the effectiveness of the electrostatic attractions from the nucleus away.
When you go down a group, electronegativity decreases because there are more shells so electrons are further away from the positive nucleus which reduces the attraction of electrons.
When you go across a period, electronegativity increases because there are more protons in the nucleus so attraction between the nucleus and electrons increase.Additionally, atoms get smaller because proton number increases and this will cause electrons to be more attracted to the nucleus and orbit it closer.
When do polar and non-polar covalent bonds occur?
Note:Bonds that are polar have a bond dipole moment-The measure of strength and direction of the polarity in the bond.(the bigger the difference in electronegativity, the bigger the bond dipole moment)
Note:C-H bonds in organic molecules are not regarded as being polar.
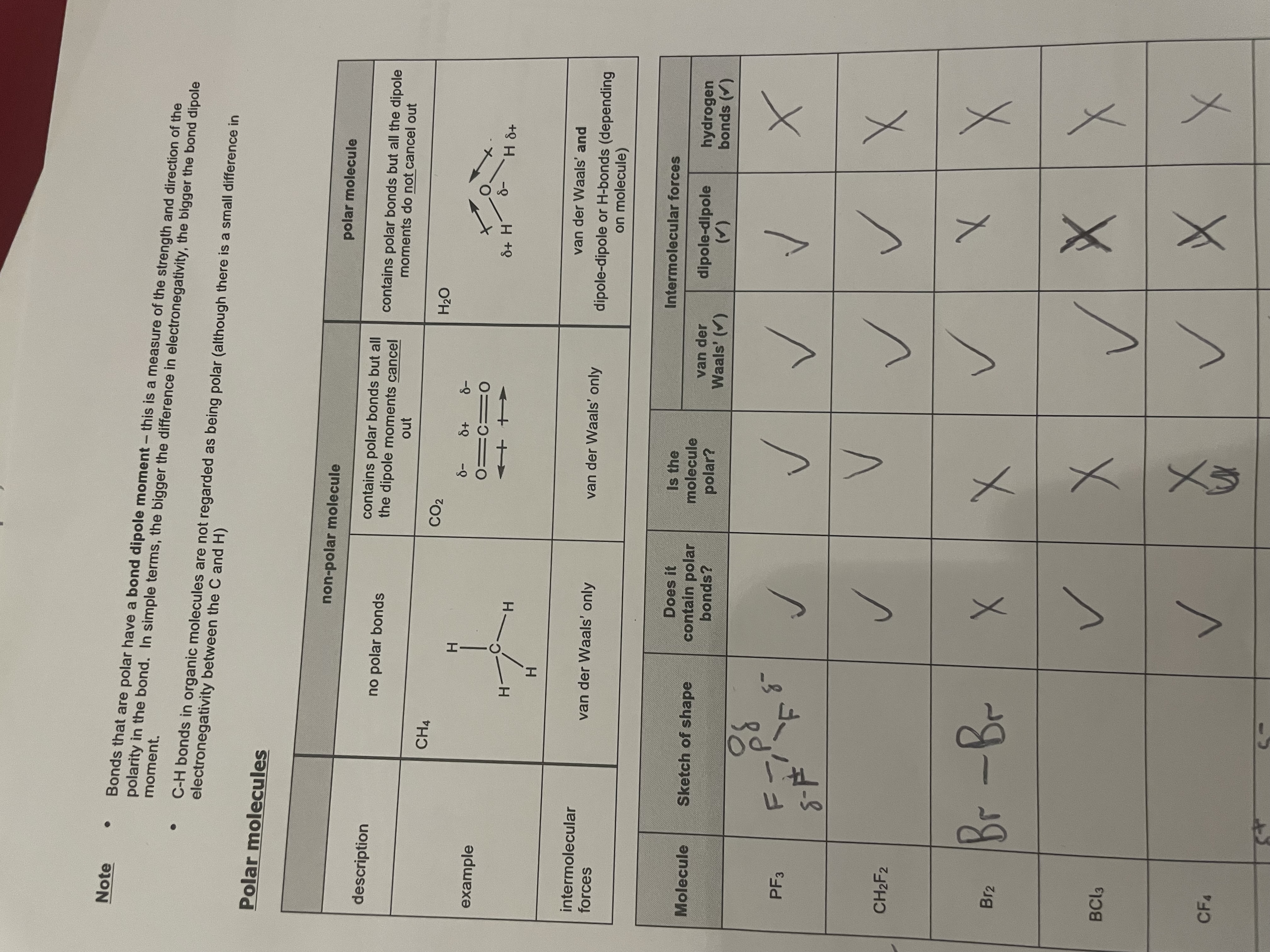
Non-Polar:When 2 atoms in a covalent bond have the same electronegativity.→A covalent bond where the 2 electrons are shared equally e.g. Cl-Cl bond in Cl2.
Polar:When the 2 atoms in a covalent bond have a different electronegativity.→A covalent bond where the 2 electrons are not shared equally.(The more electronegative atom has a greater share of the 2 electrons and is delta/slightly- whereas the other atom is less electronegative and has the lower share so is therefore delta/slightly +). E.g. H-Cl bond in HCl.
Polar molecules and non-polar molecules:
note:arrows to show whether a molecule is polar point from the positive atom to the negative. If two arrows are facing the same direction then they do not cancel out (polar molecule) but if they face opposite directions then they cancel out(non-polar molecules)
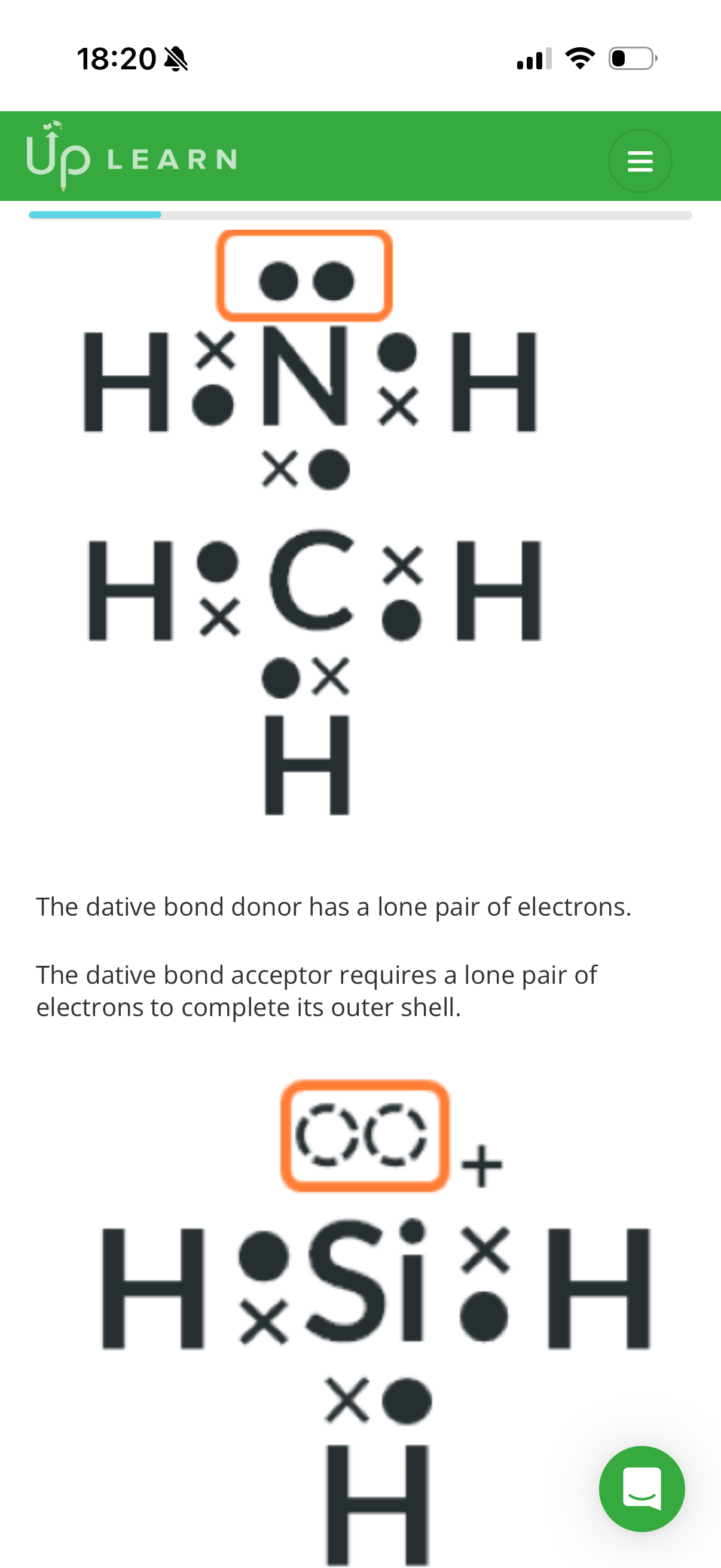
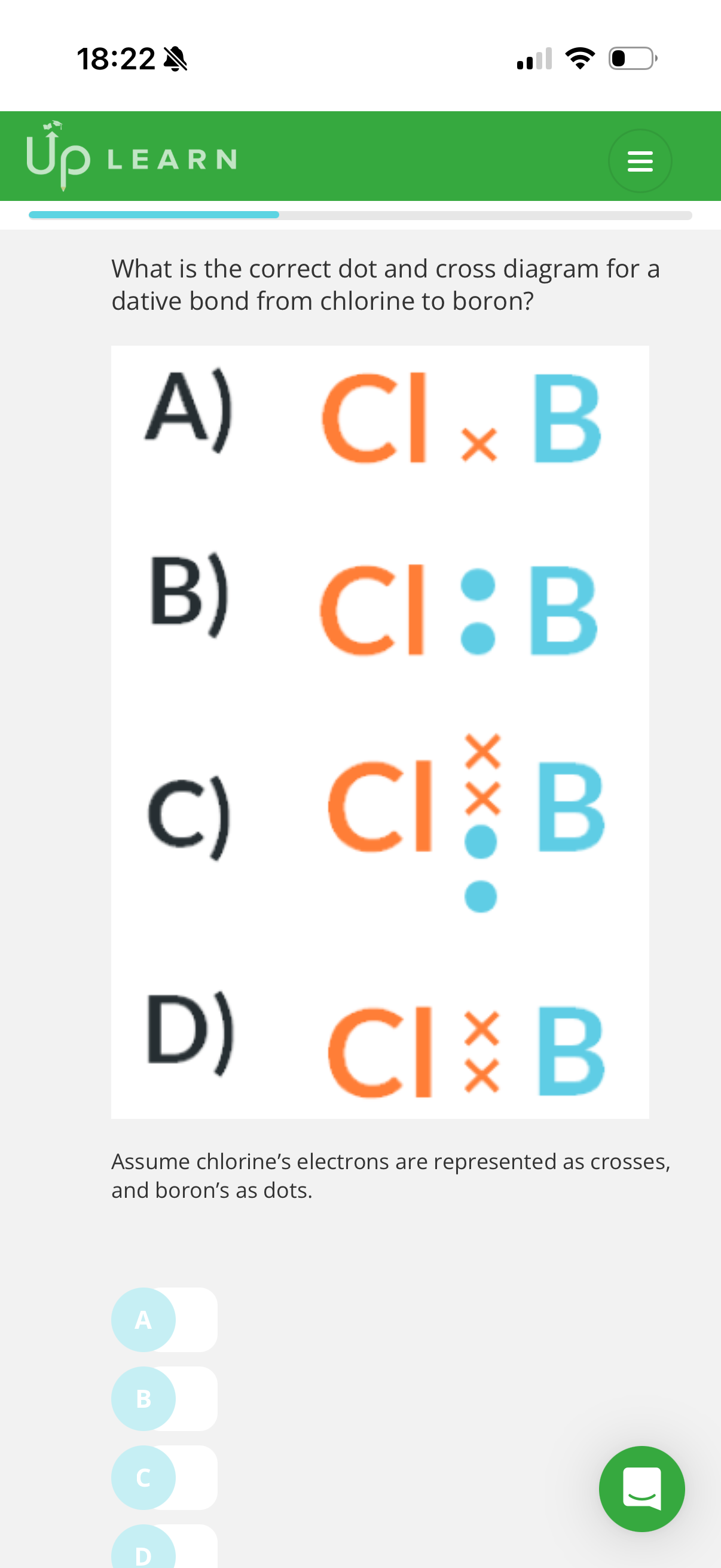
1.What is a co-ordinate bond?(dative covalent bond)
A covalent bond which both electrons in the shared pair come from the same atom
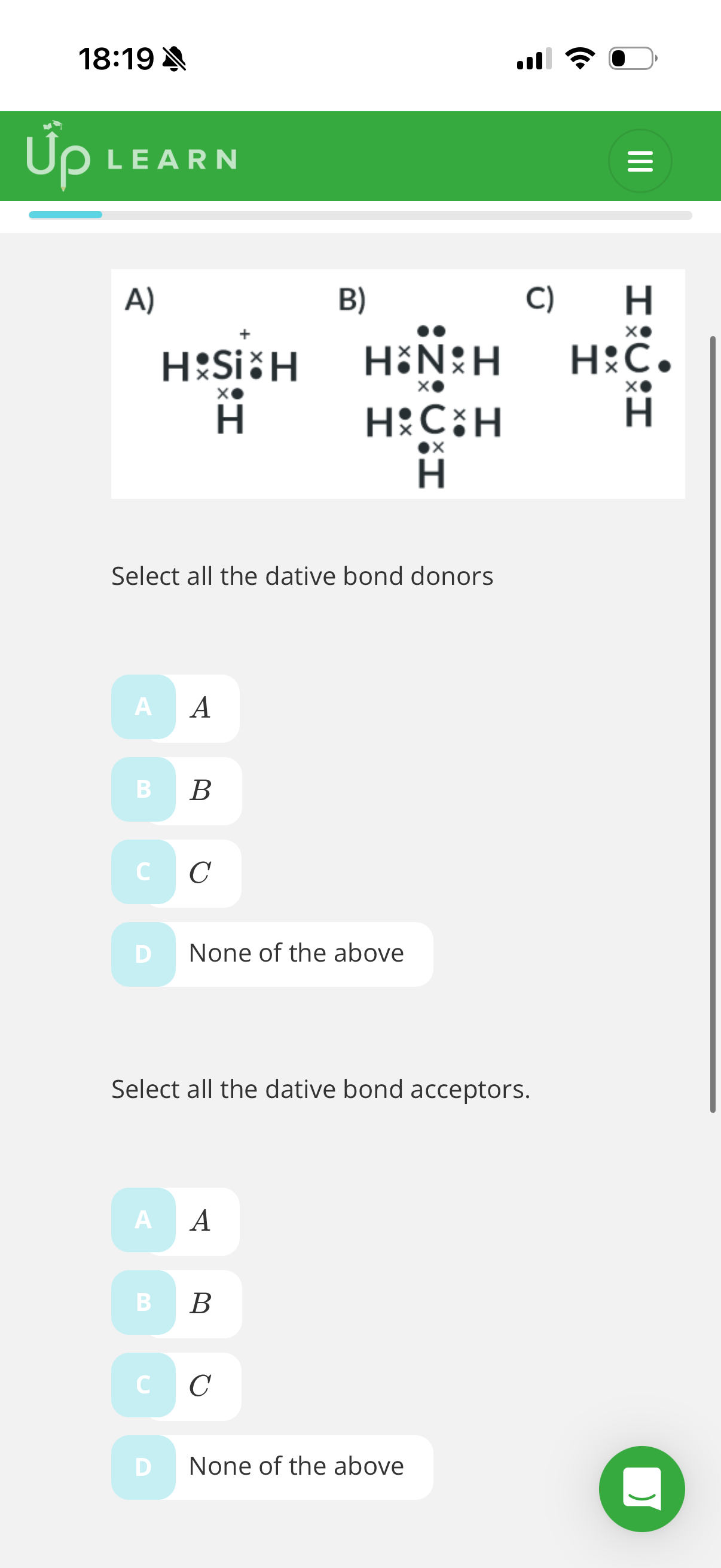
Answer image q
D then A
Answer image q?
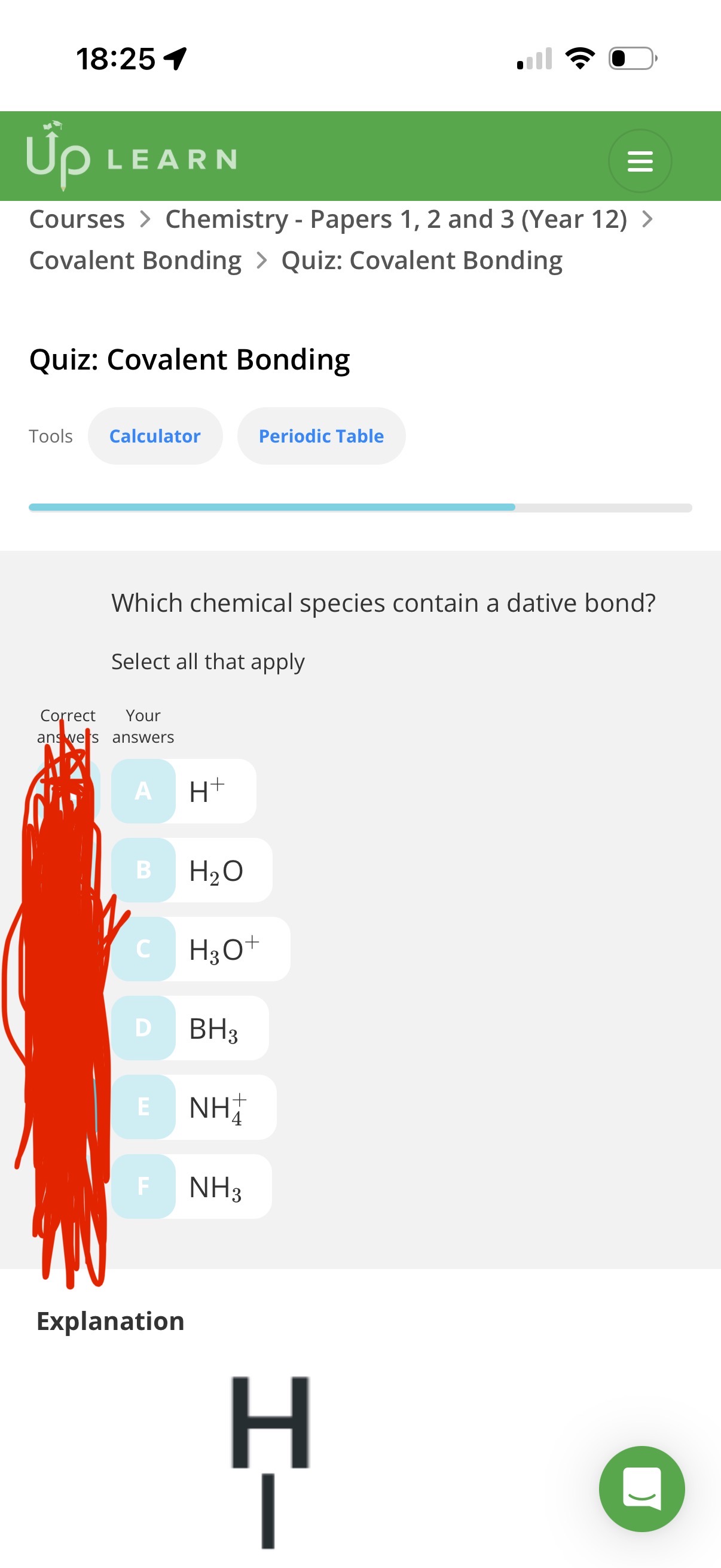
Answer image q?
(Ask teacher)
C and E
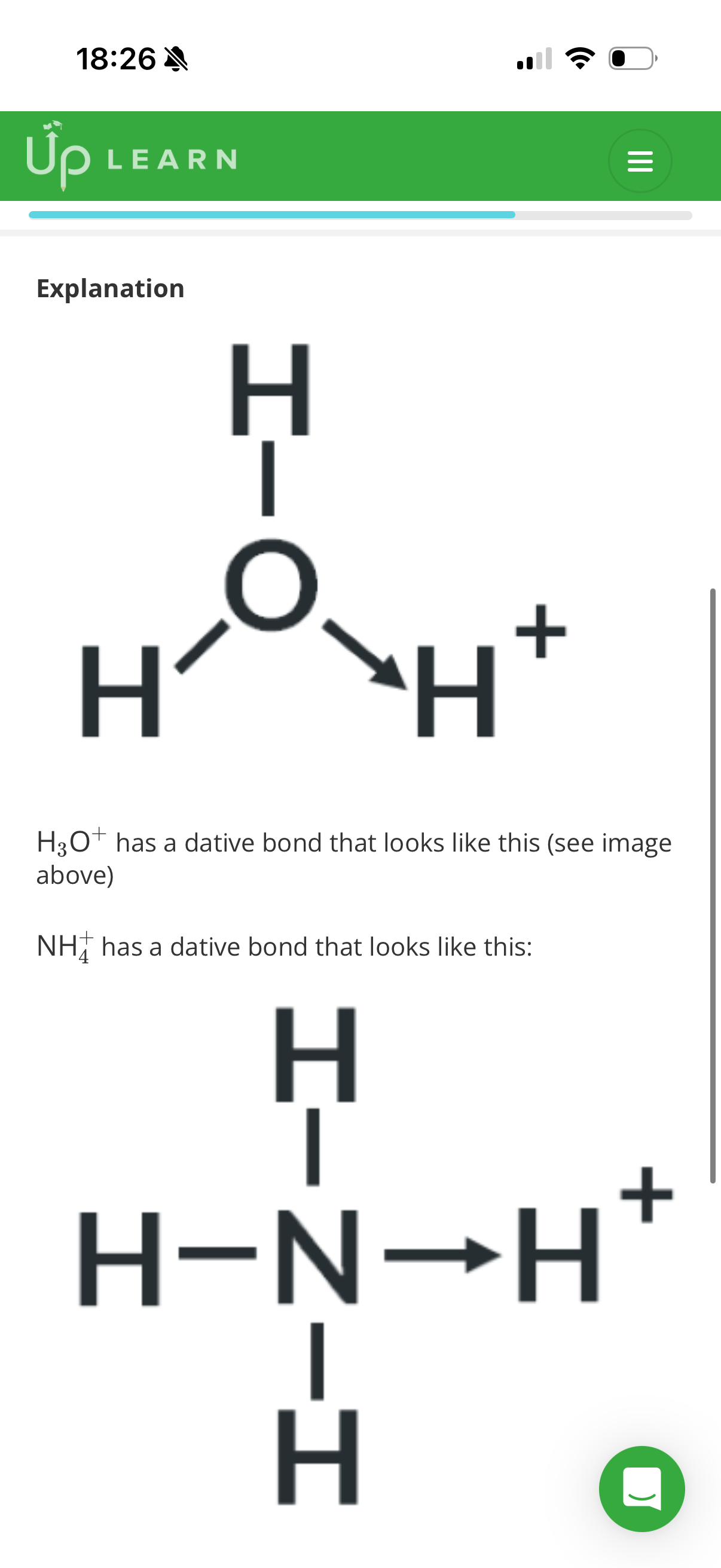
•Electrons exist in …1? within a covalent bond.
•Electron pairs that aren’t shared are called lone pairs.
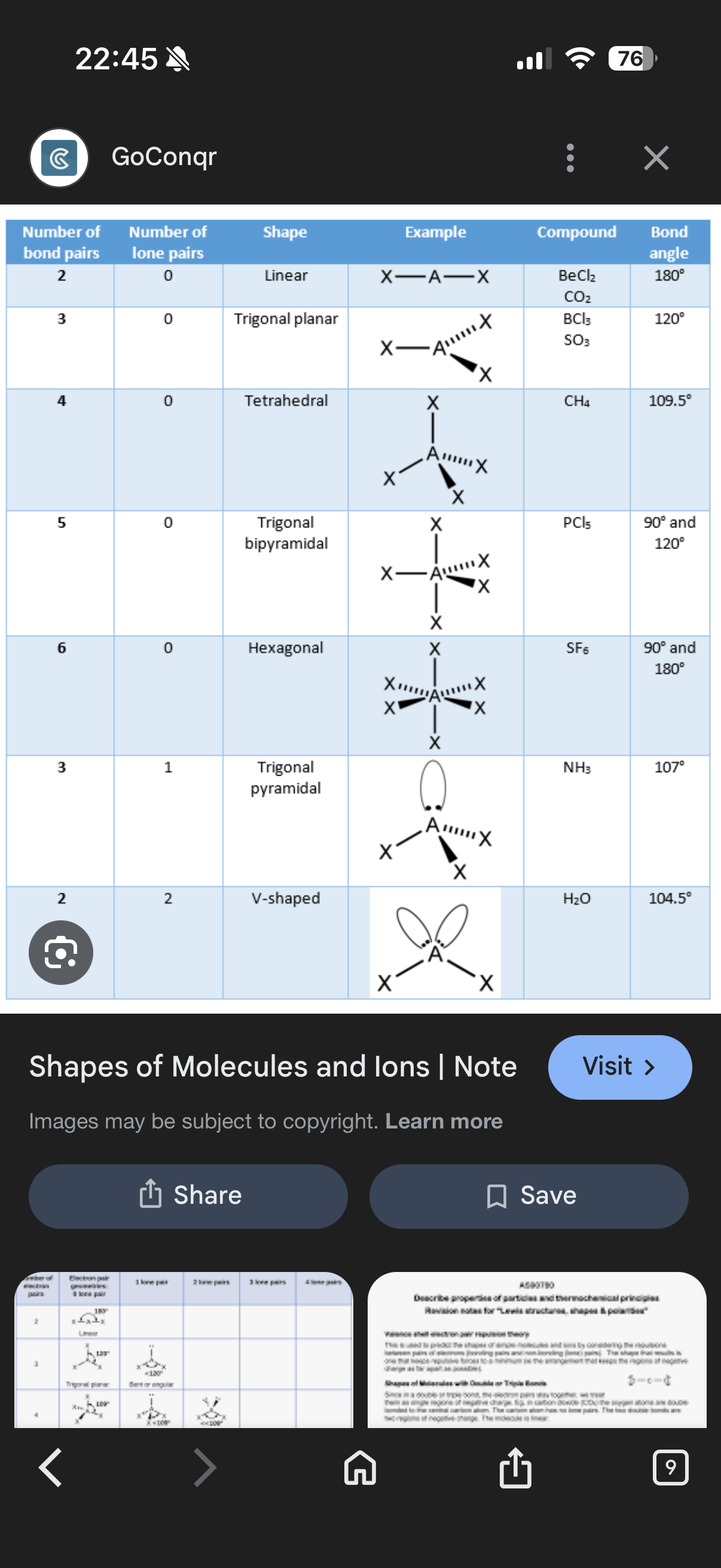
•The resulting clouds repel each other and if there are 4 pairs,the angle between each bond would be 109.5•.
•Lone pairs form a cloud that isn’t stretched between 2 atoms and will repel just a little bit more than a bonding pair(around 2.5• more)
2.If there was a lone pair in a molecule with 4 bonds such as CH4, what degrees would the angle be between the 3 other bonds?
1.pairs. 2. (109.5 - 2.5 =) 107•

Molecule shapes and how to draw them:
Note:When drawing the shapes, you should take the bonds on the equator before the poles(north and south/top and bottoms)
1.A molecule with 2 bonding pairs and 2 lone pairs has what shape and what angle?
Info: To work out the structure, you need to find the group of the first part of the word e.g. P is in group 5. You then need to look at the first word of the other element e.g. tri-oxide. Tri is 3 so 5+3=8 and so 4 electron pairs can be made. Because there’s only 3 oxygens the max bonds made is 3 and so there will be 1 lone pair. So we know that the shape will be Trigonal pyramidal.
1.It has a bent shape and its angle is 104.5•

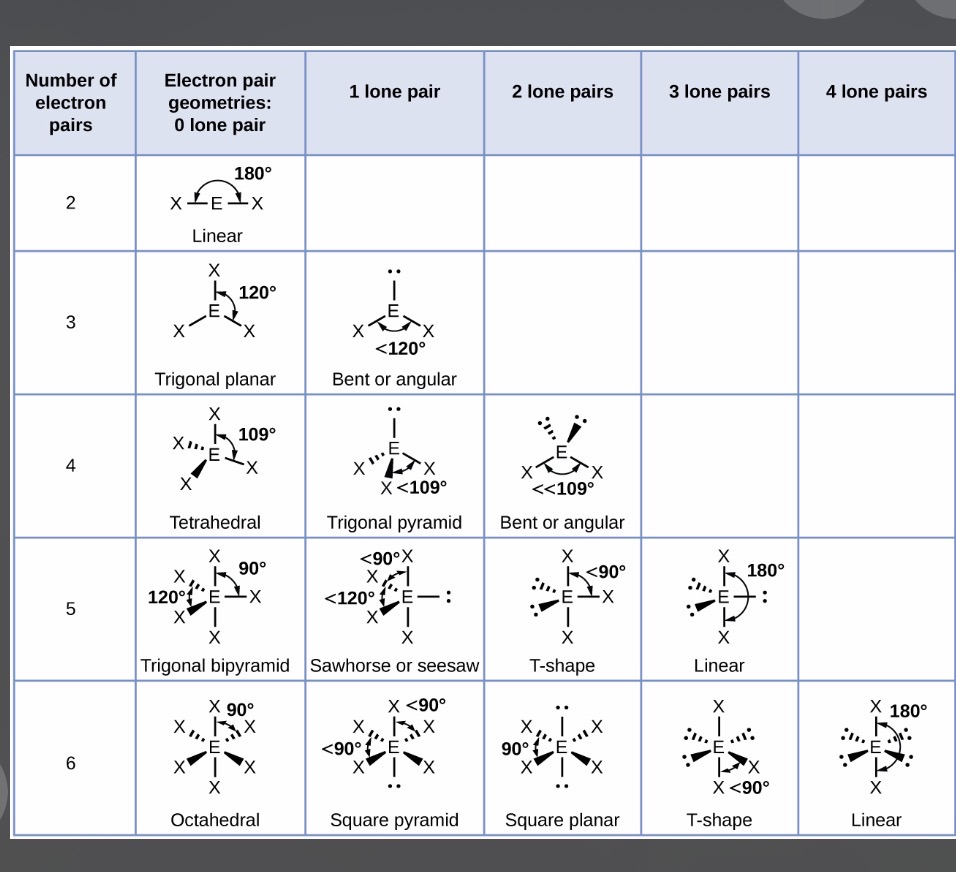
Images:
Note-The image where it has 4 pairs and 2 lone pairs is supposed to have an angle of 104.5