lec 14 - cell metabolism and beyond (zong)
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
metabolism
the breakdown and synthesis of macromolecules in living cells
macromolecules include:
lipids
nucleic acid
carbs
proteins
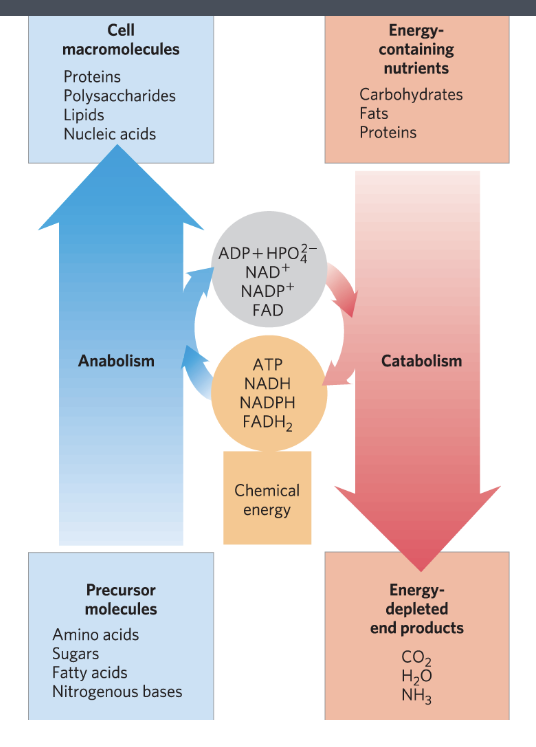
catabolism and anabolism
catabolism = the degradative phase of metabolism → releases energy
anabolism = the building phase of metabolism → requires energy
connected by carrier molecules that store energy and electrons
ATP
NADH
NADPH
FADH2
anabolism
precursor molecules
AA
sugars
fatty acids
nitrogenous bases
goes through anabolism which involves carrier molecules and chemical energy
ATP → ADP + HPO42-
NADPH → NAD+
NADH → NADP+
FADH2 → FAD
results in cell macromolecules
proteins
polysaccharides
lipids
nucleic acids
catabolism
energy-containing nutrients
carbs
fats
proteins
goes through catabolism which involves energy being released as the macromolecules are broken down and that energy is used to regenerate carrier molecules
ADP + HPO42- → ATP
NAD+ → NADH
NADP+ → NADPH
FAD → FADH2
results in energy-depleted end products
CO2
H2O
NH3
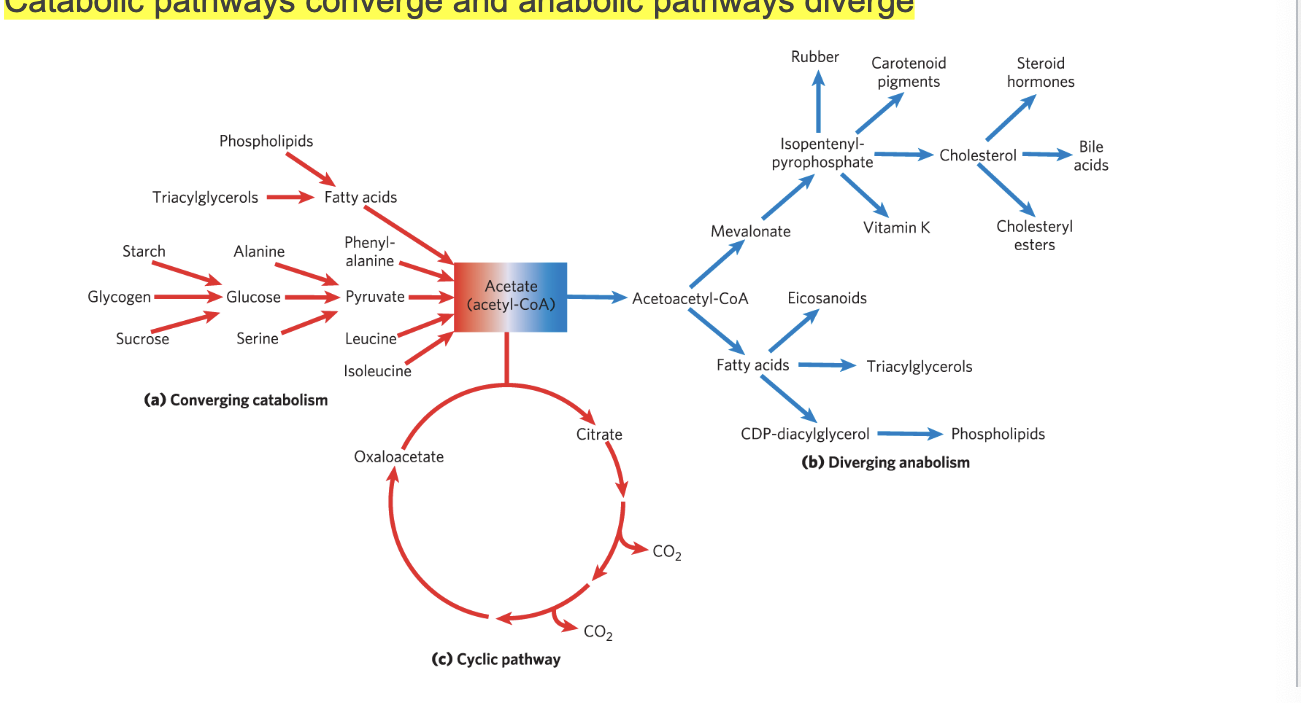
catabolic pathways vs anabolic pathways
catabolic pathways converge and anabolic pathways diverge
complex molecules are broken down into simple building blocks which can then be used to build complex molecules
diagram
for catabolism
multiple diverse molecules (starch, glycogen, sucrose) are broken down into common intermediate acetyl-coA → enters TCA cycle for further oxidation to CO2, oxaloacetate (allows it to keep running)
for anabolism
from common intermediate, acetyl-coA many biosynthetic pathways:
fatty acids → triacylglycerols
mevalonate → cholesterol → bile acids, steroid hormones
isopentenyl-pyrophosphate → carotenoids, vit K, rubber
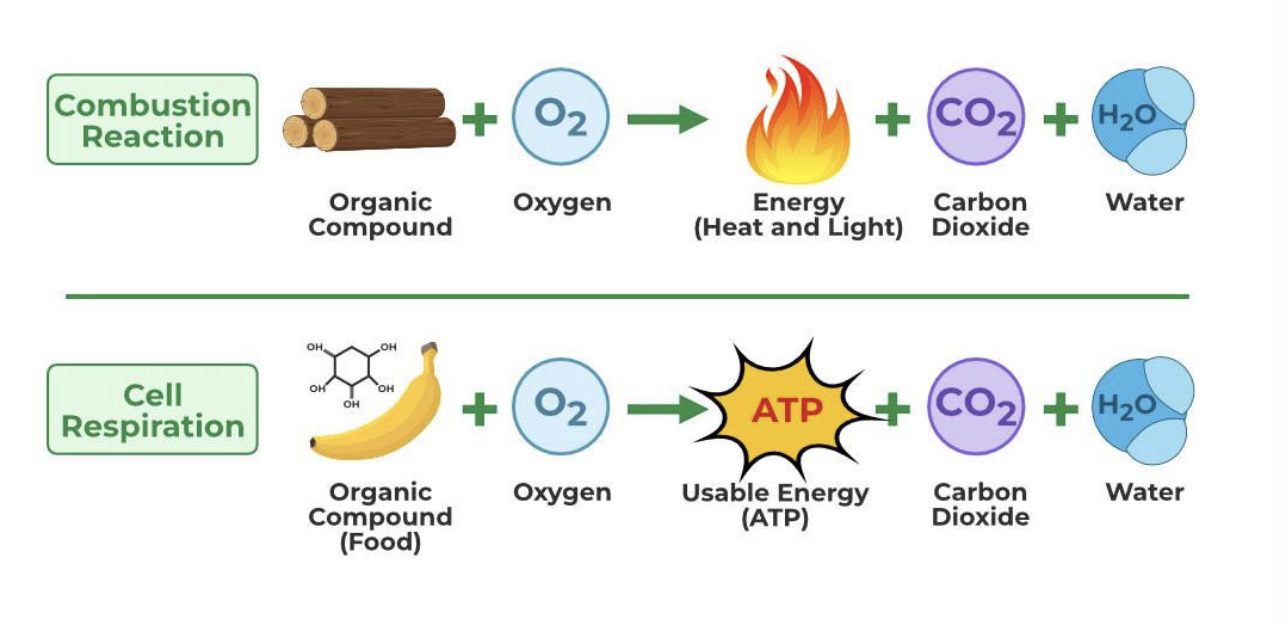
catabolism: energy production
combustion rxn
organic compound + O2 → energy (heat and light) + CO2 + H2O
all energy is released quickly and uncontrollably → NOT usable by cells
cell respiration
organic compound (food) + O2 → ATP + CO2 + H2O
energy is released gradually and stored in ATP which powers biological functions
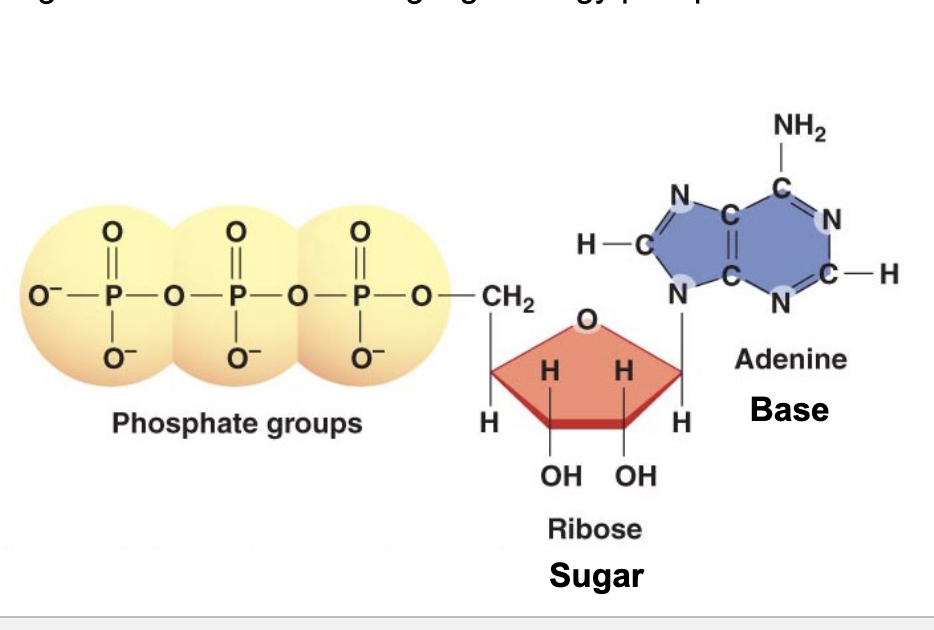
ATP
adenosine triphosphate → “energy cash” in the cell
organic molecule containing high energy phosphate bonds
structure
3 phosphate groups → each is negatively charged
breaking one of these bonds releases energy used to fuel cellular processes
ribose sugar
adenine base
sugar + base = adenosine
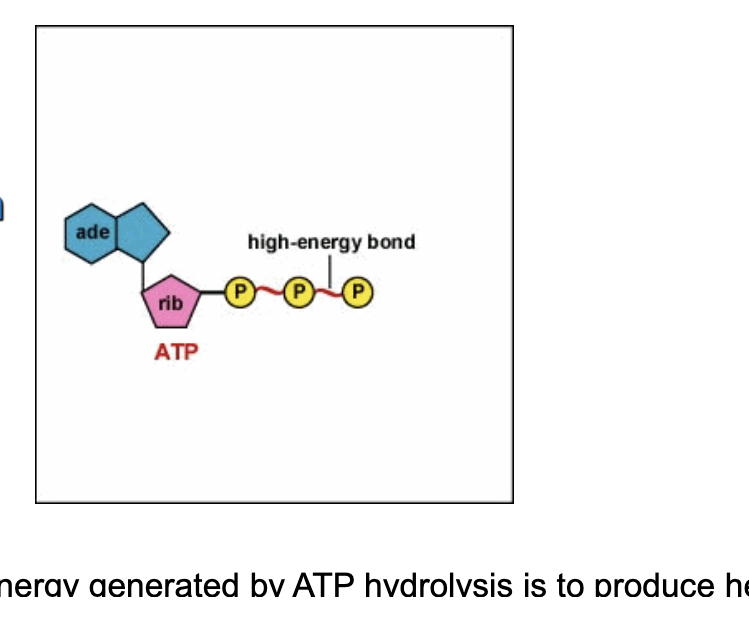
how do we get energy from ATP?
by breaking the high energy bonds between the last 2 phosphates in ATP
in humans, ~60% energy generated by ATP hydrolysis is to produce heat
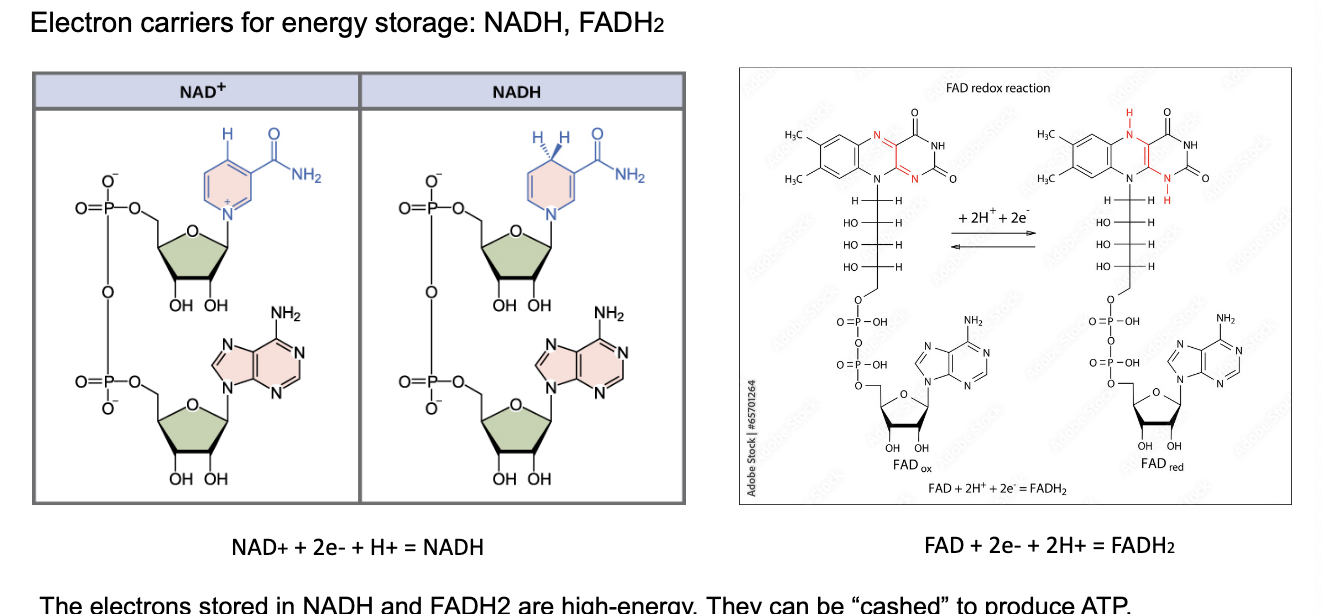
electron carrier for energy storage: NADH, FADH2
the electrons stored in NADH and FADH2 = high energy → can be 'cashed’ to product ATP
NADH
NAD+ + 2e- + H+ = NADH (reduced form that holds energy)
FADH2
FAD + 2e- + 2H+ = FADH2
analogy
ATP = energy ‘cash’
NADH and FADH2 = energy ‘bonds’ (savings that can be converted to cash)
bioenergy comes from redox reaction: transfer of electrons
electron donor = being oxidized
THINK: OIL RIG
oil = oxidation is loss of electrons → donating electrons
electron receiver = being reduced
THINK: opposite of reduction is gain → gain of electrons
energy is released when electrons are transferred from high energy state to low energy state; this energy can be harnessed by pumping H+ to generate proton gradient then producing ATP
examples
Mg + Cl2 → Mg2+ + 2Cl-
Mg is oxidized → loses 2 e-
Cl is reduced → each Cl atom gains 1 e-
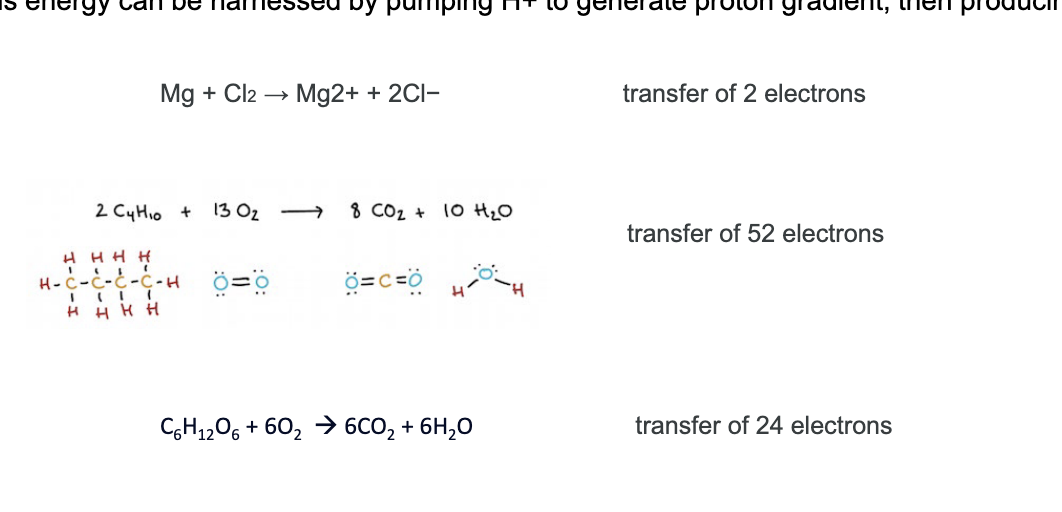
glucose metabolism
steps
glucose enters the cell from the blood via a glucose transporter
in the cell, hexokinase converts glucose into glucose-β-phosphate
glucose-β-phosphate is broken down into pyruvate which produces 2 ATP and H+
H+ leaves cell through sodium-hydrogen exchanger and into blood
pyruvate has 2 pathways
aerobic pathway (with O2)
pyruvate enters the mitochondria and undergoes: pyruvate oxidation, TCA, electron transport chain → final output = 36 ATP + H+
HCO3- is a byproduct → goes through anion exchanger → into blood
anaerobic pathway (without O2)
pyruvate → lactate → regenerates NAD+, allowing glycolysis to continue → lactate transporter out of cell using mono-carboxylate transporter → into blood
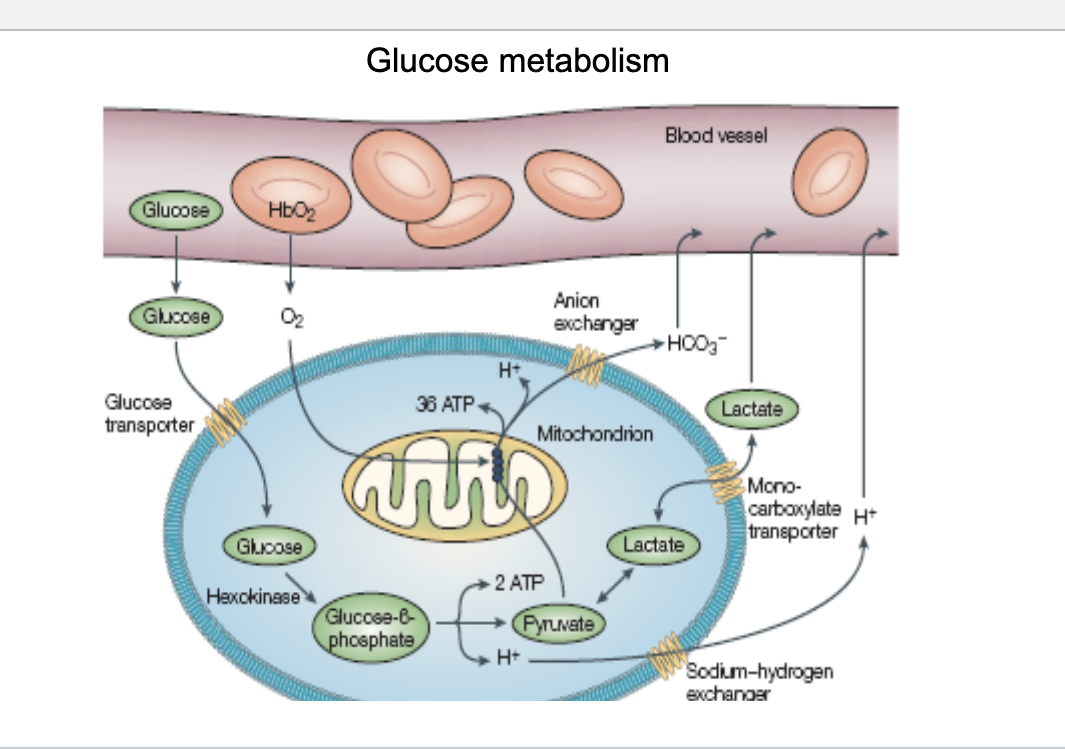
glucose transporters
general note
lower kM = higher affinity
bloog glucose level roughly 3.9-8.3 mM
GLUT1
tissue location
all other mammalian tissue
Km
1 mM
responsive to insulin
NO
comments
basal glucose uptake in erythrocytes and brain
GLUT2
tissue location
liver
also pancreatic β cells
kidney cells
Km
15-20 mM
responsive to insulin
NO
comments
in liver, removes excess glucose from the blood
also used for export of glucose from liver
GLUT3
tissue location
all other mammalian tissues
Km
1 mM
responsive to insulin
NO
comments
major glucose transporter in neurons
GLUT4
tissue location
muscle and fat cells
Km
5 mM
responsive to insulin
YES
comments
expression in muscle plasma membrane increases with endurance training
you need insulin 4 GLUT 4 in your 4 limbs of muscle and fat
GLUT5
tissue location
small intestine
Km
n/a
responsive to insulin
NO
comments
primarily a fructose transporter
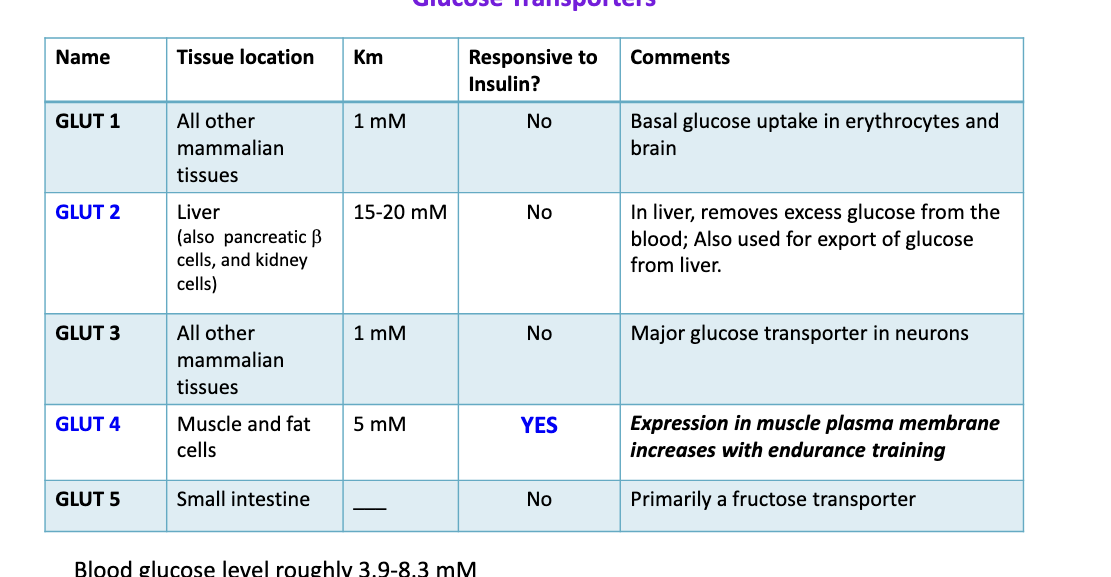
10 steps of glycolysis
steps 1 and 3 = investment steps to prepare glucose to be split (use ATP)
steps 7 and 10 = substrate level of phosphorylation to generate ATP
prep phase = 1-5
priming glucose molecules with phosphate groups to make it easier to split → uses 2 ATP
payoff phase = 6-10
extract energy
overview of glycolysis
oxidation and cleavage of glucose
in all cells
the hub of carbohydrate metabolism b/c virtually all carbohydrates can be converted to glucose
occurs in the cytosol
converts 6-C glucose → 3-C pyruvate
net products = 2 pyruvate, 2 NADH, 2 ATP
phase I: energy investment
6-C glucose phosphorylated using 2 ATP → fructose-1,6-biphosphate → splits into two 3-C molecules called glyceraldehyde 3-P
phase II: energy payoff
glyceraldehyde-3-P → phosphate added from Pi (free floating phosphate present in cytosol) → 1,3-biphosphoglycerate → NAD+ converted to NADH + H+ and 2 ADP converted to 2 ATP → pyruvate
4 ATP produced but net 2 ATP b/c u consumed 2 in phase 1
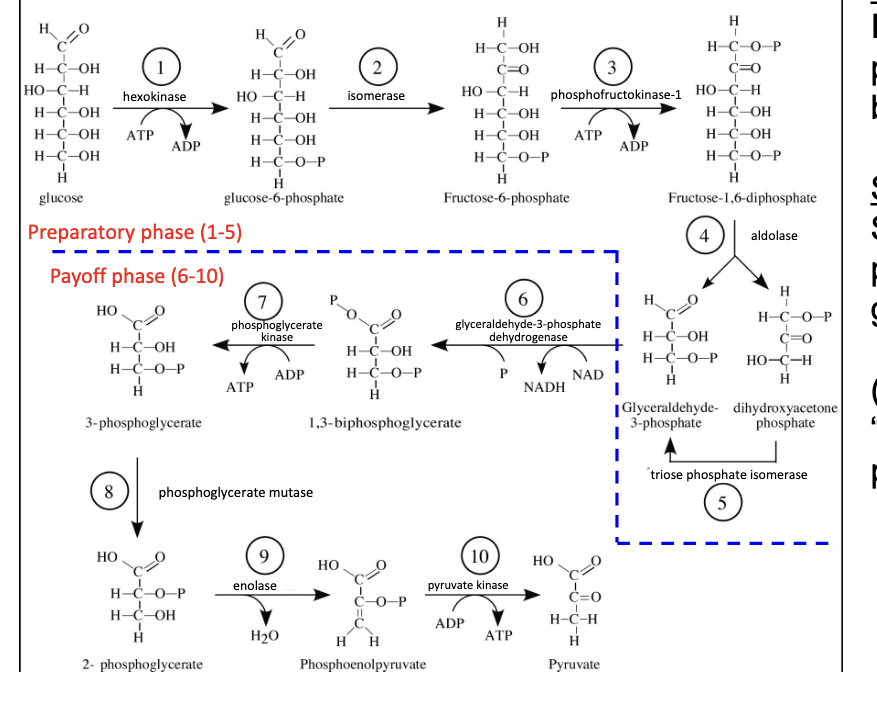
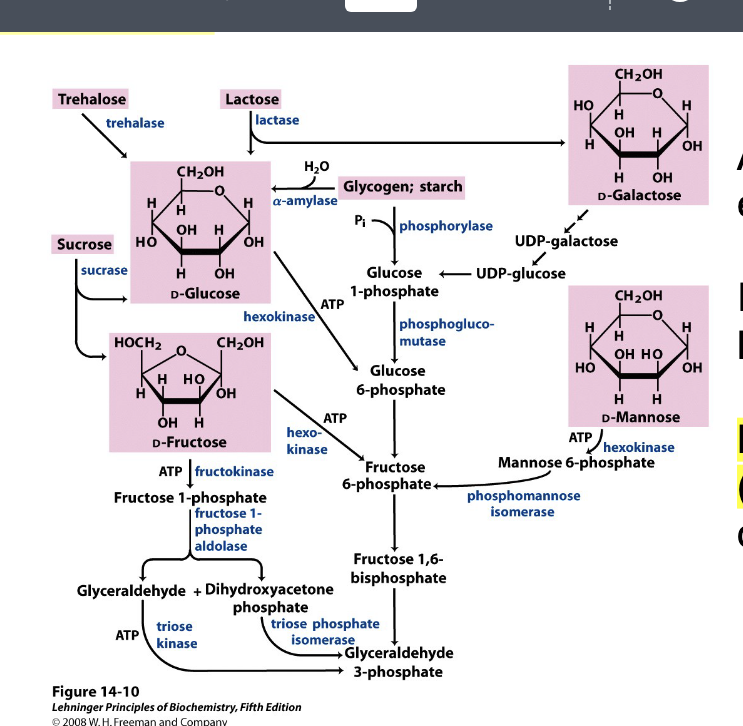
feeder pathways
all carbohydrates enter glycolysis
in muscle, often via hexokinase
lactose intolerance = lactase deficiency
not enough lactase available to convert lactose into D-galactose which then gets made into intermediates for glycolysis
image shows how variety dietary carbs are funneled into glycolysis → all carbs must be converted into intermediates of glycolysis like G-6-phosphate to be metabolized for energy
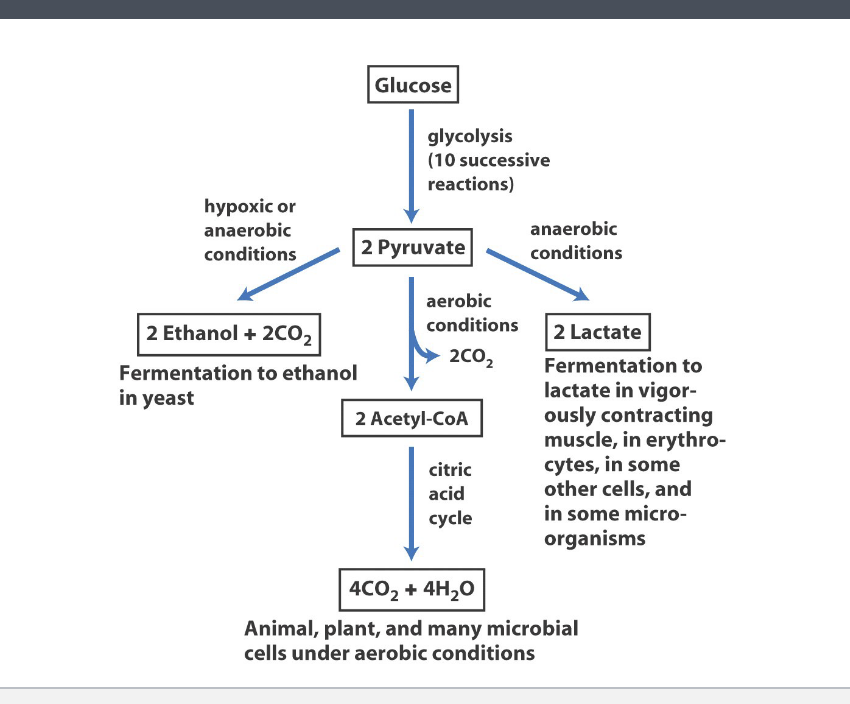
fate of the products: pyruvate and NADH
glucose → glycolysis (10 successive reactions) → 2 pyruvate
aerobic conditions: animal, plant, and many microbial cells under aerobic conditions
2 pyruvate enters mitochondria → 2 CO2 is released as its converted to 2 acetyl-coA → acetyl coA enters the citric acid cycle (TCA) → produces 4 CO2 + 4 H2O
anaerobic conditions: fermentation to lactate in vigorously contracting muscle, in erythrocytes, in some other cells and in some other micro-organisms
2 pyruvate → 2 lactate
hypoxic or anaerobic conditions: fermentation to ethanol in yeast
2 pyruvate → 2 ethanol + 2 CO2
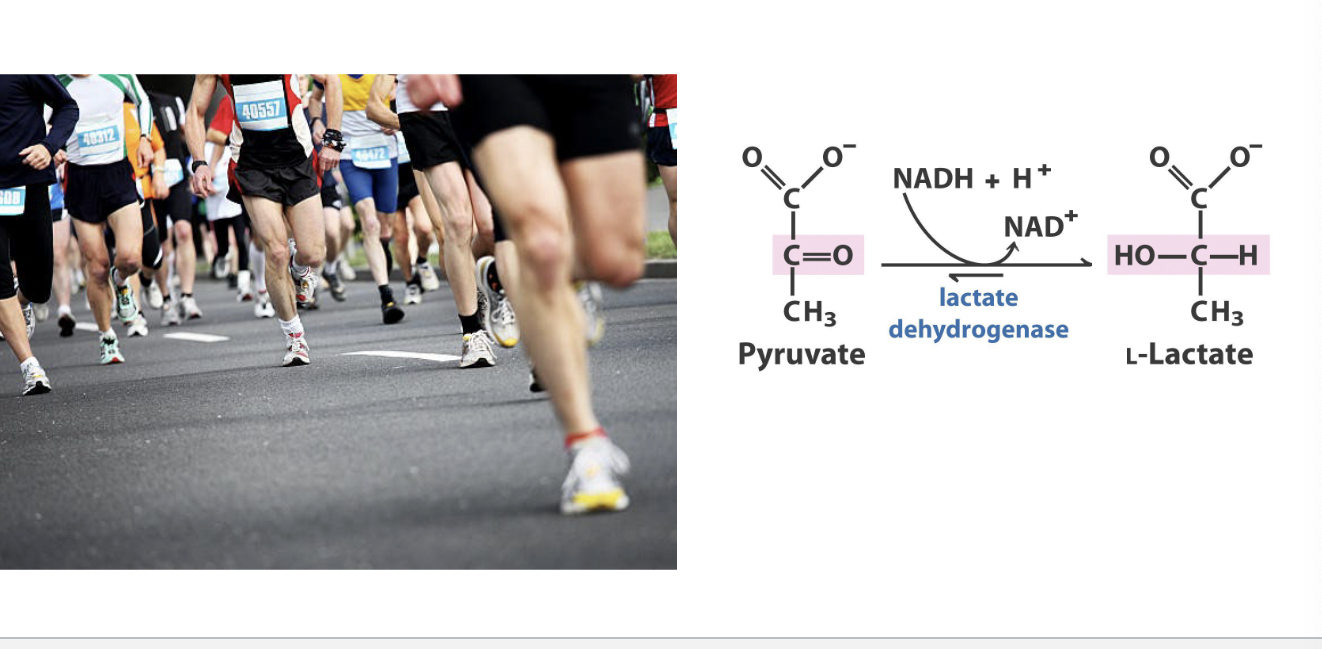
fermentation in animals
intense exercise → anaerobic metabolism and lactate production
muscle consume oxygen quickly, sometimes faster than its deliver → creates hypoxic or anaerobic conditions in muscle
pyruvate + NADH + H+ → lactate + NAD+
through lactate dehydrogenase
regenerates NAD+ to keep glycolysis going (CANNOT make ATP without it)
allows short-term ATP production even WITHOUT oxygen
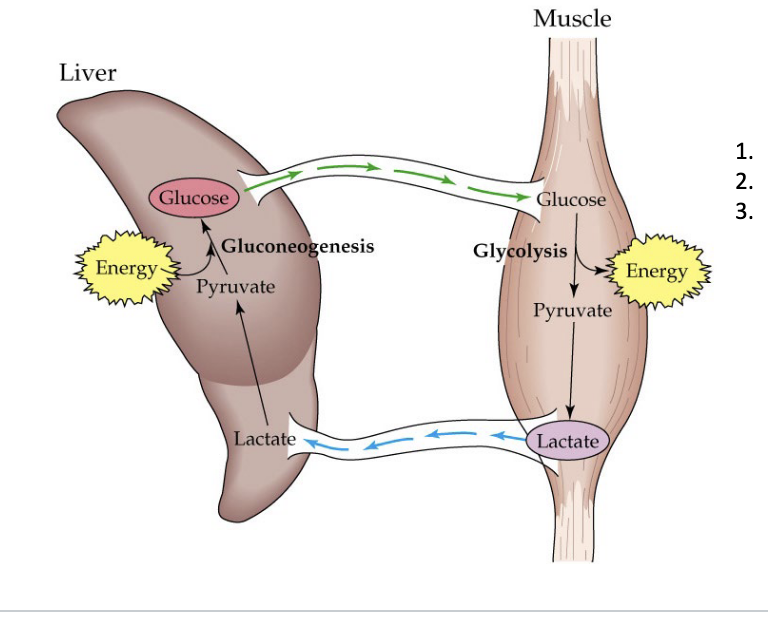
cori cycle
lactate produced by anaerobic glycolysis in muscles gets transferred into the liver where lactate is used for glucose production then sent back to muscles
purpose
facilitate muscle exertion
prevents lactate acidosis
main source of gluconeogenesis during fasting
diagram
muscle
glucose goes through glycolysis and creates energy in muscle → generates pyruvate → lactate
liver
lactate goes to liver → becomes pyruvate → expends energy to create glucose in process called gluconeogenesis → glucose goes back to muscle so that it can continue glycolysis to create energy
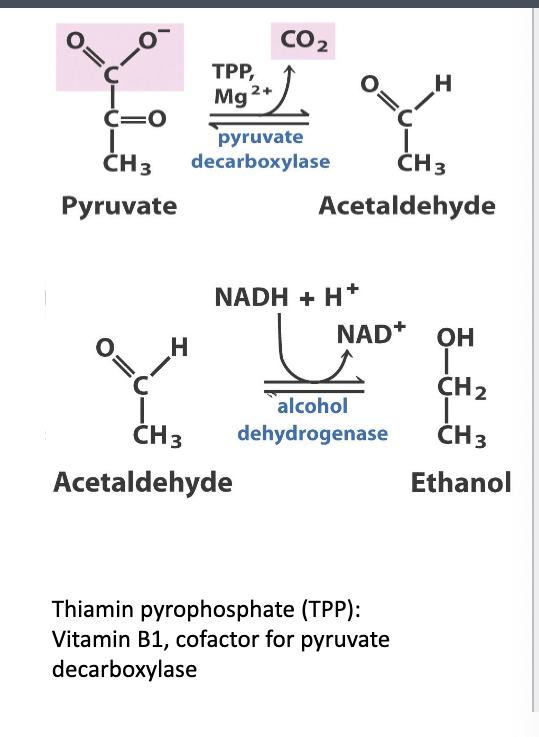
fermentation in yeast
example = beer
pyruvate → acetaldehyde + CO2
enzyme catalyzing rxn = pyruvate decarboxylase
cofactor = thiamine pyrophosphate (TPP) and Mg2+
acetaldehyde → ethanol
enzyme= alcohol dehydrogenase
NADH + H → NAD+
uses this to reduce acetaldehyde
regenerates NAD+ to keep glycolysis running
NAD+ is coupled to ATP which is why its regeneration is important
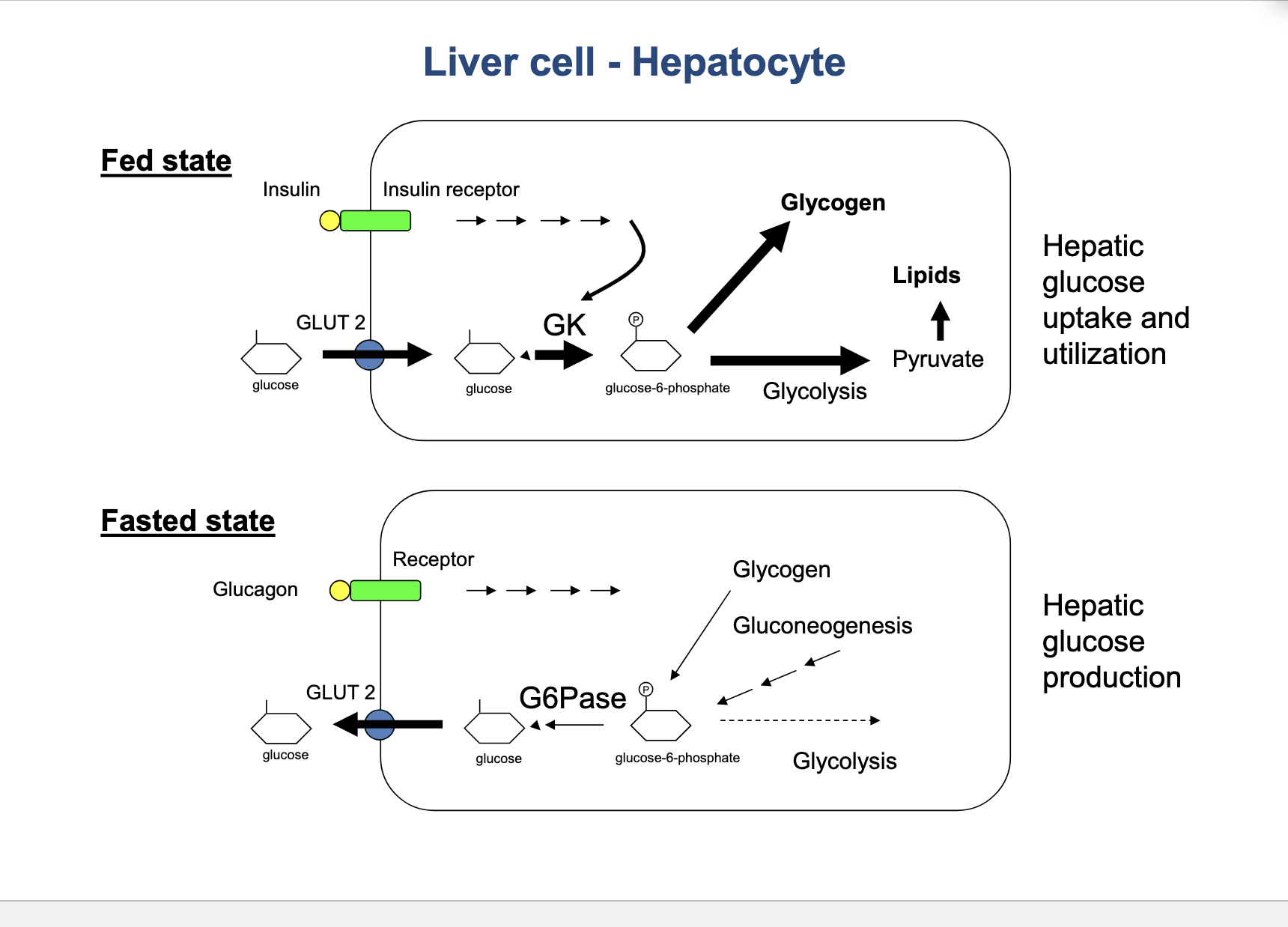
liver cell - hepatocyte
fed state → hepatic glucose uptake and utilization
insulin is released from pancreas in response to high blood glucose and binds to insulin receptor of hepatocyte and stimulates GK
glucose is brought into cell via GLUT2 transporter → glucokinase (GK) phosphorylates → glucose-6-phosphate
→ glycolysis → pyruvate → lipid synthesis
glycogen (storage form of glucose)
fasted state
glucagon is secreted when blood glucose is low and binds to receptor of hepatocyte signals to glycogen
glycogen → release glucose-6-phosphate → G6Pase converts glucose-6-phosphate into free glucose → transported through GLUT2 out into blood
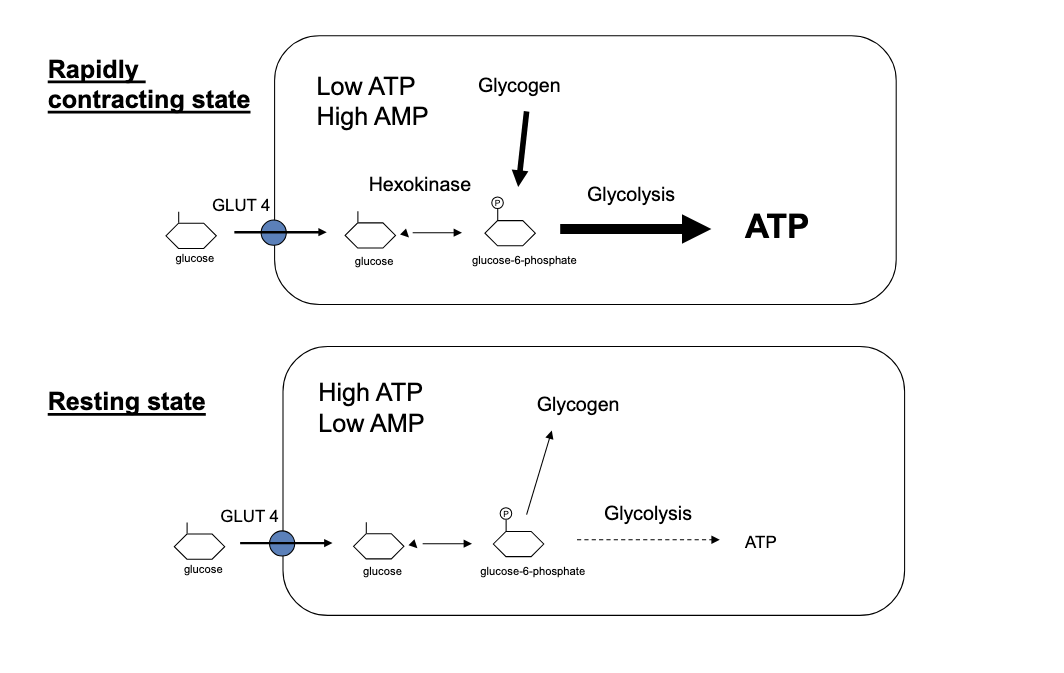
skeletal muscle cell
rapidly contracting state (e.g. exercising)
low ATP/ high AMP (signal of low energy)
glucose is brought into cell via GLUT4 transporters → hexokinase converts glucose to glucose-6-phosphate → glycolysis → ATP
also stored glycogen is broken down into G6P to further fuel glycolysis
outcome = max ATP production to meet urgent energy demand of muscle contractions
resting state
high ATP/low AMP
glucose is brought into cell via GLUT4 transporters → converted to G6P → instead of fueling glycolysis, channeled towards glycogen synthesis (storage)
→ also has glycolysis but it slows down b/c cell already has enough ATP
outcome = energy is conserved and glucose is stored as glycogen for future use
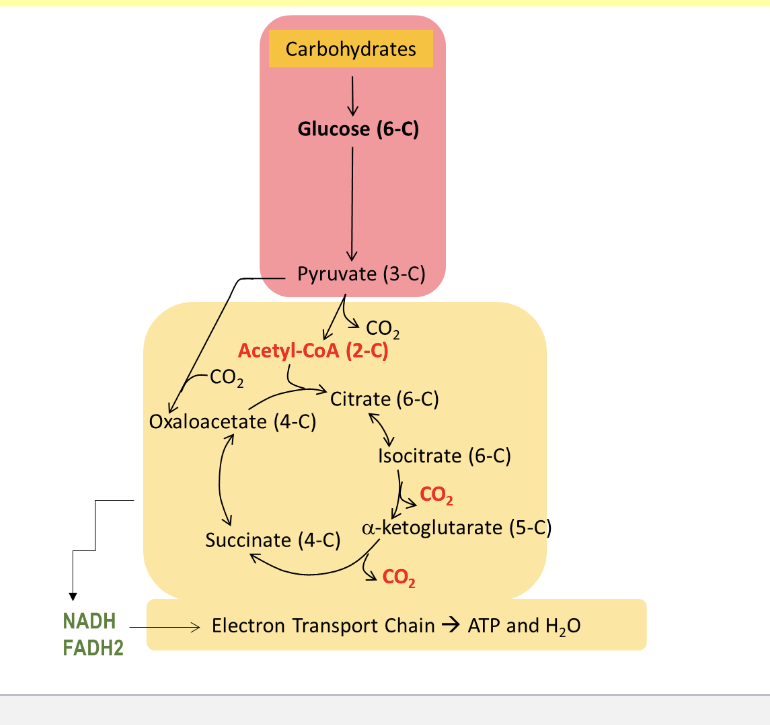
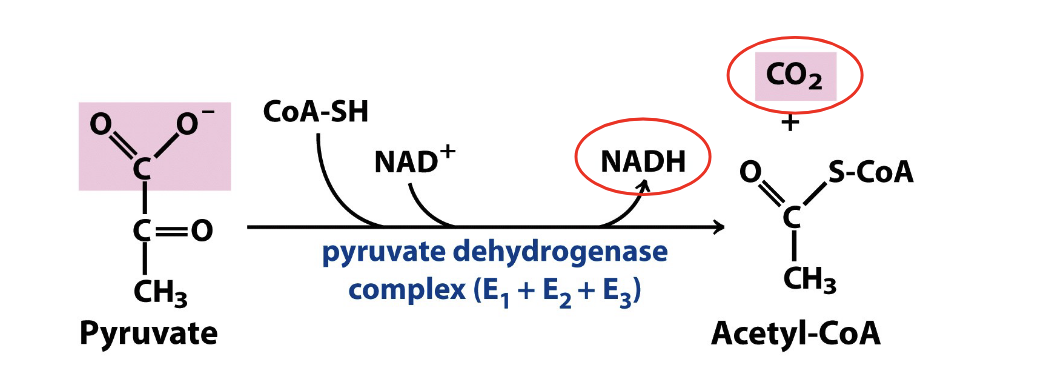
pyruvate enters mitochondria to be further oxidized
carbs get converted to glucose and then to pyruvate where it enters the mitochondria and goes thru TCA cycle
PDHC catalyzes the oxidative decarboxylation of pyruvate
product of oxidative decarboxylation of pyruvate is acetyl coA
irreversible rxn
produces NADH
releases the first free CO2
thiamin pyrophosphate (TPP) = coenzyme
inhibited by arsenic
pyruvate → acetyl coA
enzyme = pyruvate dehydrogenase complex
NAD+ → NADH
CoA-SH attaches to the acetyl group to form acetyl-coA
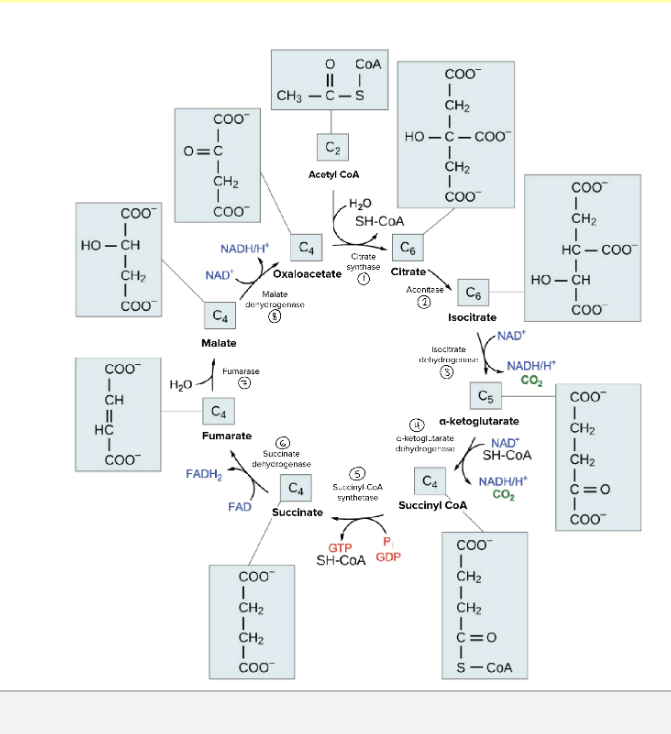
overview of the TCA cycle
occurs in the mitochondria
occurs in all organs except those that lack mitochondria
aerobic pathway
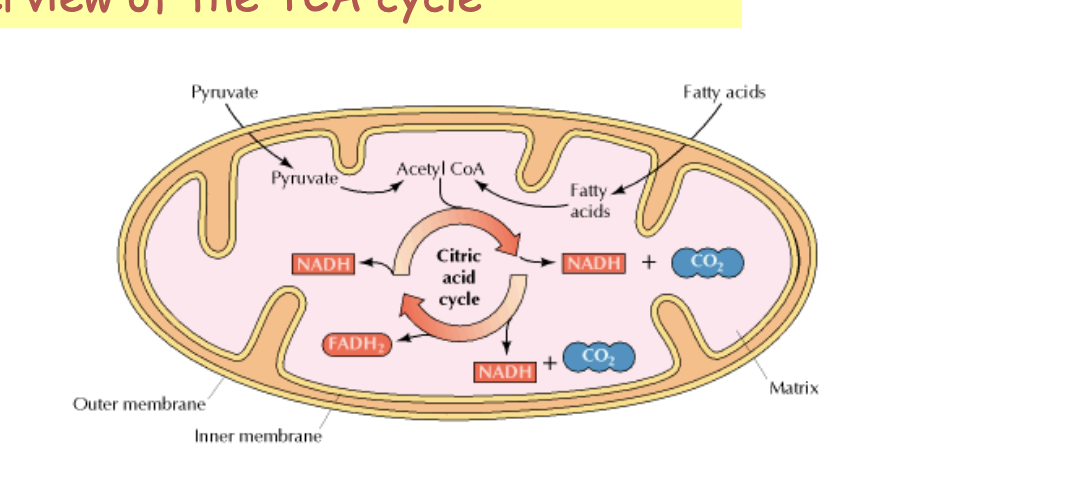
main functions of the TCA (tri-carboxylic acid) cycle
citric acid oxidizes the 2 carbons of acetyl coA → CO2
in the process of oxidation, high-energy electrons are captured in the form of NADH and FADH2
key function = harvest high-energy electrons from carbon fuels which can then be used by the electron transport chain (ETC) for oxidative phosphorylation
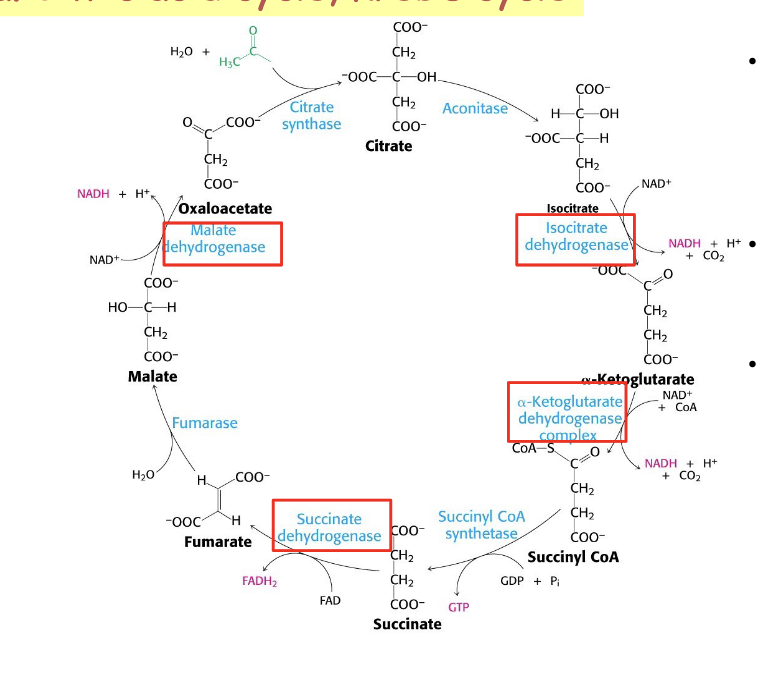
TCA cycle
in step 1 of TCA cycle, 2 carbon acetyl CoA condenses with 4-carbon oxaloacetate to form 6-carbon citrate through citrate synthase
although oxygen is NOT directly involved in any of the steps, TCA cycle is tightly coupled to the ETC and oxidative phosphorylation and is thus dependent on O2
4 distinct classes of dehydrogenases in the TCA cycle = responsible for electron capture to generate NADH and FADH2
isocitrate dehydrogenase
produce CO2 → literally just take it off the molecule
α-ketoglutarate dehydrogenase complex
produce CO2 → literally just take it off the molecule
succinate dehydrogenase
gives free electrons to FAD → FADH2
forms double bond
malate dehydrogenase
gives free electrons to NAD → NADH
forms double bond
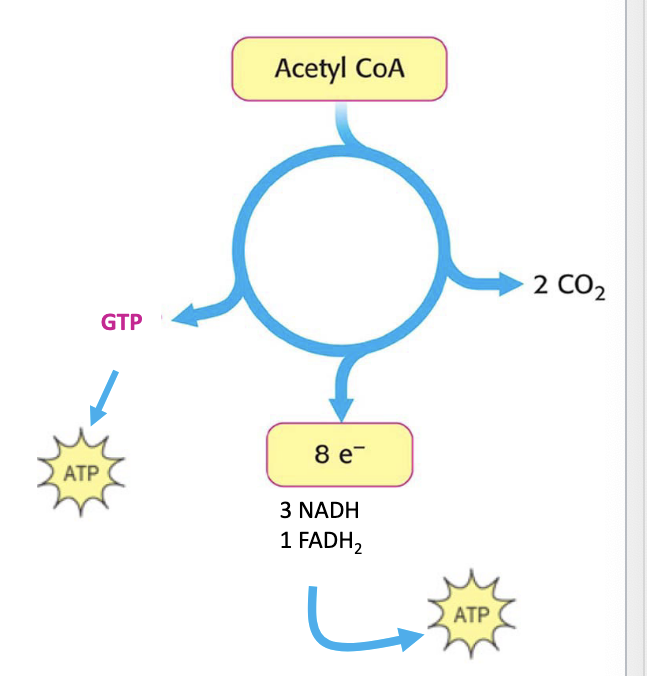
1 turn through TCA produces:
3 NADH molecules
1 FADH2 molecules
1 GTP molecule
releases 2 molecules of CO2
cellular energy levels determine the rate of TCA cycle
when cellular energy charge is high, TCA cycle is inhibited
high levels of NADH (NADH is used to make ATP in ETC) → slows down TCA cycle and PDH activity
when cellular energy charge is low, TCA cycle is activated
lots of muscle activity → high Ca2+ levels → stimulates TCA cycle and PDH activity
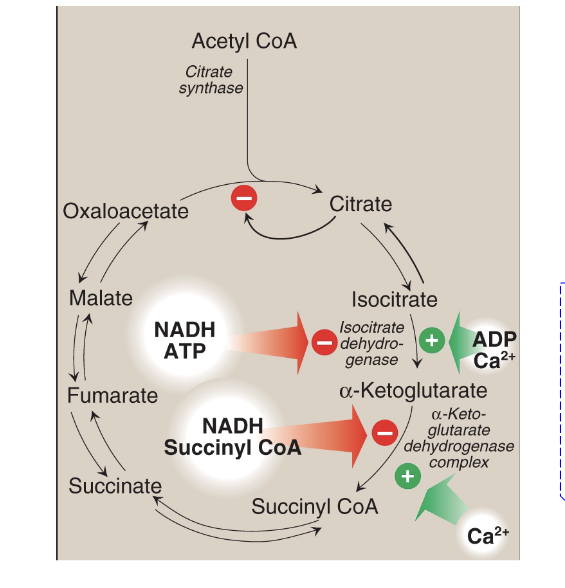
TCA cycle intermediates can participate in both catabolic and anabolic rxns
many of the intermediates of the TCA cycle participate in other metabolic reactions
energy and metabolic needs of the cell dictate the direction of the rxns
since intermediates of the TCA cycle can participate in both catabolic and anabolic reactions → reactions are described as anaplerotic
example
citrate → fatty acids or steroids
alpha-ketoglutarate → glutamate → other AA → purines
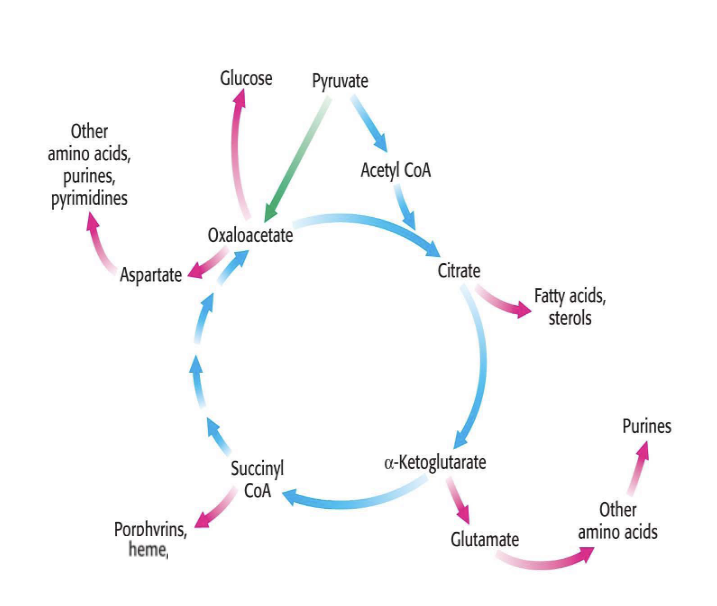
anaplerosis
chemical reactions that form intermediates of a metabolic pathway
examples of such are found in the TCA cycle
in the normal function of the TCA cycle for respiration, concentrations of TCA intermediates remain constant
however, many biosynthetic reactions also use these molecules as substrate → in the context of the TCA cycle, anaplerosis = process of replenshing intermediates that have bene extracted for biosynthesis
energy yielded from the TCA cycle
starting with acetyl-coA the final reaping of 1 TCA cycle is as follows
3 NADH → 3 NAD+ → 9 ATP
FADH2 → FAD → 2 ATP
GDP + Pi → GTP
in total = 12 ATP/acetyl coA oxidized
energy “cashing out” by oxidative phosphorylation in mitochondria
TCA is in mitochondrial matrix
oxidative phosphorylation = ETC + chemiosmosis
occurs in inner mitochondrial membrane
you eject H+ into intermembrane space which then flows back into the mitochondrial matrix to generate ATP
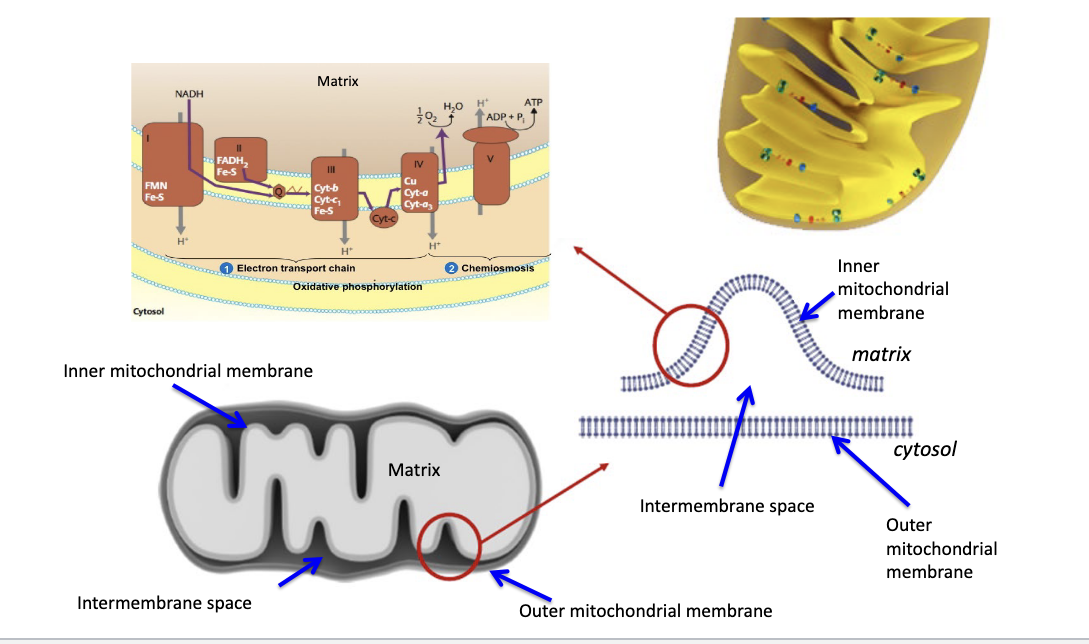
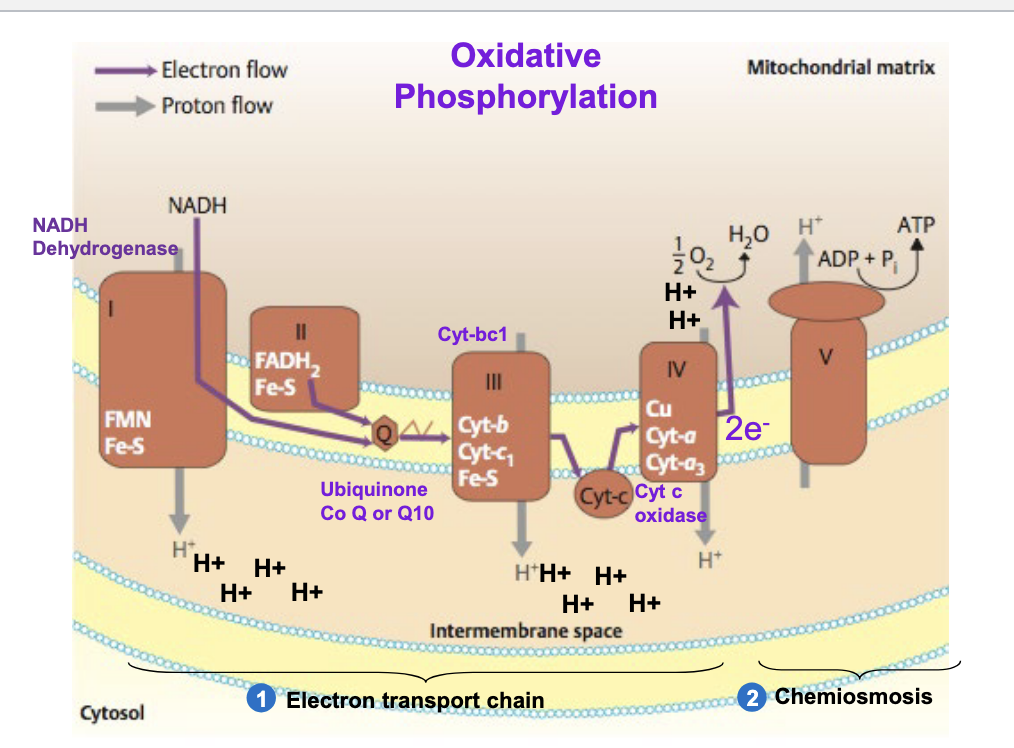
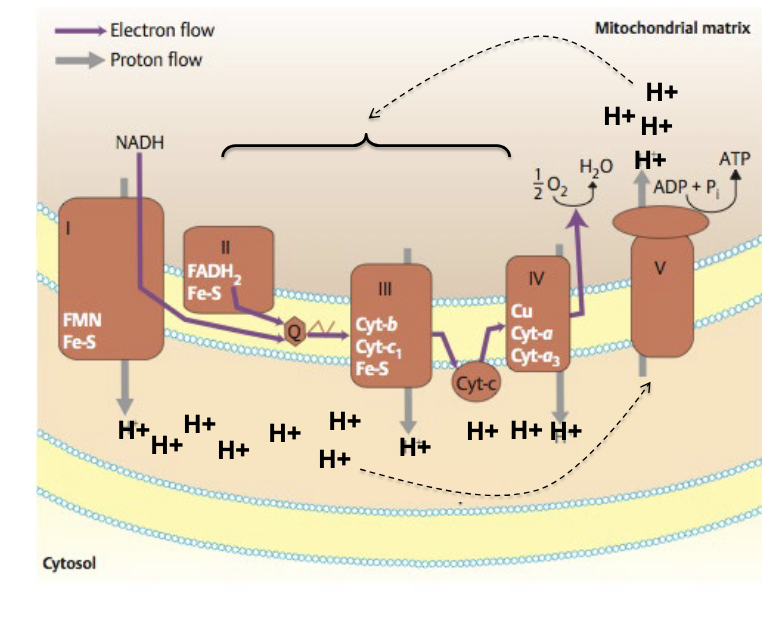
oxidative phosphorylation
5 total complexes
complex I = NADH dehydrogenase
NADH → NAD+, donates e- to FMN and Fe-S
complex II = succinate dehydrogenase
feeds electrons via FADH2
complex III = cytochrome bc1 = cytochrome reductase
passes e- to Cyt C
complex IV = cytochrome c oxidase
transfers e- to O2 → forms H2O
complex V = ATP synthase
ADP + Pi → ATP
chemiosmosis
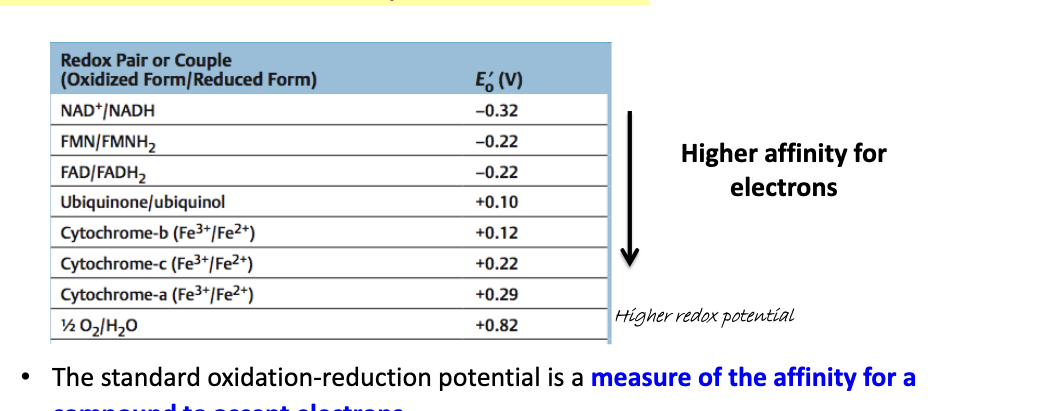
standard oxidation-reduction potential, E’o
the more positive the E’o value → higher affinity for electrons
the standard oxidation-reduction potential is a measure of the affinity for a compound to accept electrons
also called redox potential
a redox pair with a higher Eo has a higher affinity for electrons than a redox pair with lower Eo
redox pair with higher Eo will “take” electrons from a redox pair with a lower Eo
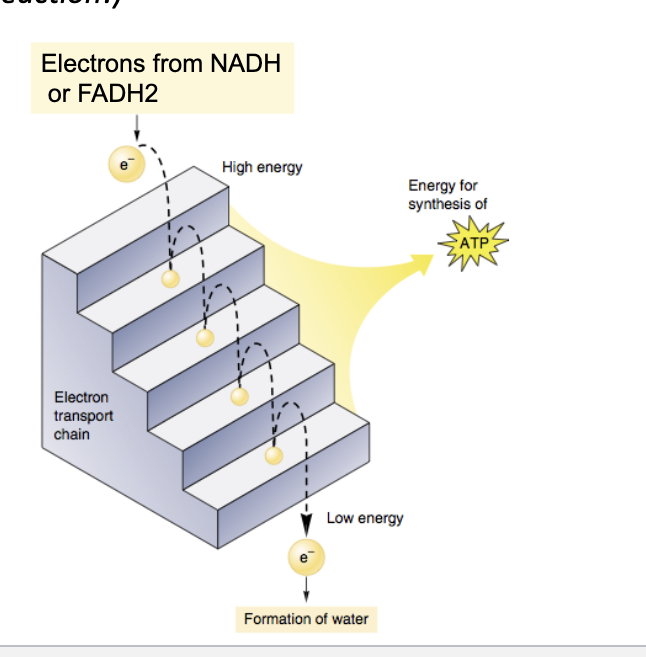
in the ETC, electrons from NADH and FADH2 are transferred step wise to a series of electron acceptors
electrons from NADH and FADH2 move in a stepwise fashion through the ETC towards oxygen → passing to lower and lower energy states and releasing energy at each step
enables cell to maximize the harvesting of energy from the ETC to drive ATP production
so without the chain it would be a huge, wasteful burst of energy but with ETC its small manageable steps where lots of ATP is made
O2 pulls electrons down the chain in an “energy-yielding tumble”
in oxidative phosphorylation, chemiosmosis couples ETC to ATP synthesis
the energy stored in a H+ ion gradient across the inner mitochondrial membrane is used to synthesize ATP
the H+ gradient is referred to as proton-motive force highlighting its capacity to do work
some energy is lose as heat → plays role in maintaining body temp
diagram
F1 = in the mitochondrial matrix contains the catalytic activity
Fo = in the inner mitochondrial membrane contains the H+ pore
chemical potential inside matrix = alkaline
electrical potential inside matrix = negative
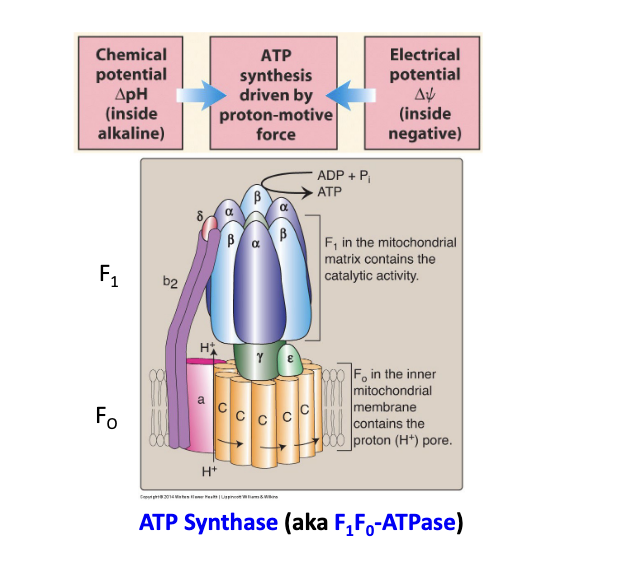
ATP synthesis is coupled to ETC
cyanide = complex IV inhibitor → blocks electron transfer to oxygen which halts electron flow, H+ pumping, ATP production
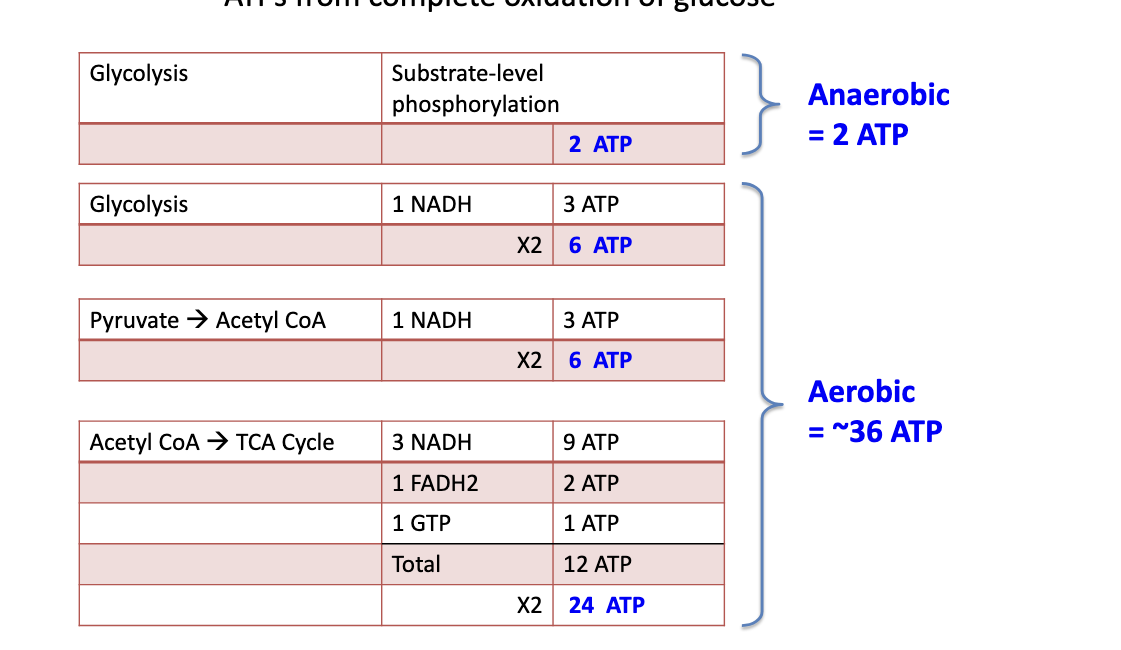
ATP from complete oxidation of glucose
anaerobic
glycolysis → 2 ATP
aerobic
glycolysis → 1 NADH generates 3 ATP → 2 NADH so 6 ATP
pyruvate → acetyl coA
1 NADH generates 3 ATP → 2 NADH so 6 ATP
acetyl coA → TCA cycle
3 NADH → 9 ATP
1 FADH2 → 2 ATP
1 GTP → 1 ATP
total 12 ATP but whole thing goes through twice because 2 pyruvate molecules so 2 acetyl coA
total for aerobic = 36 ATP
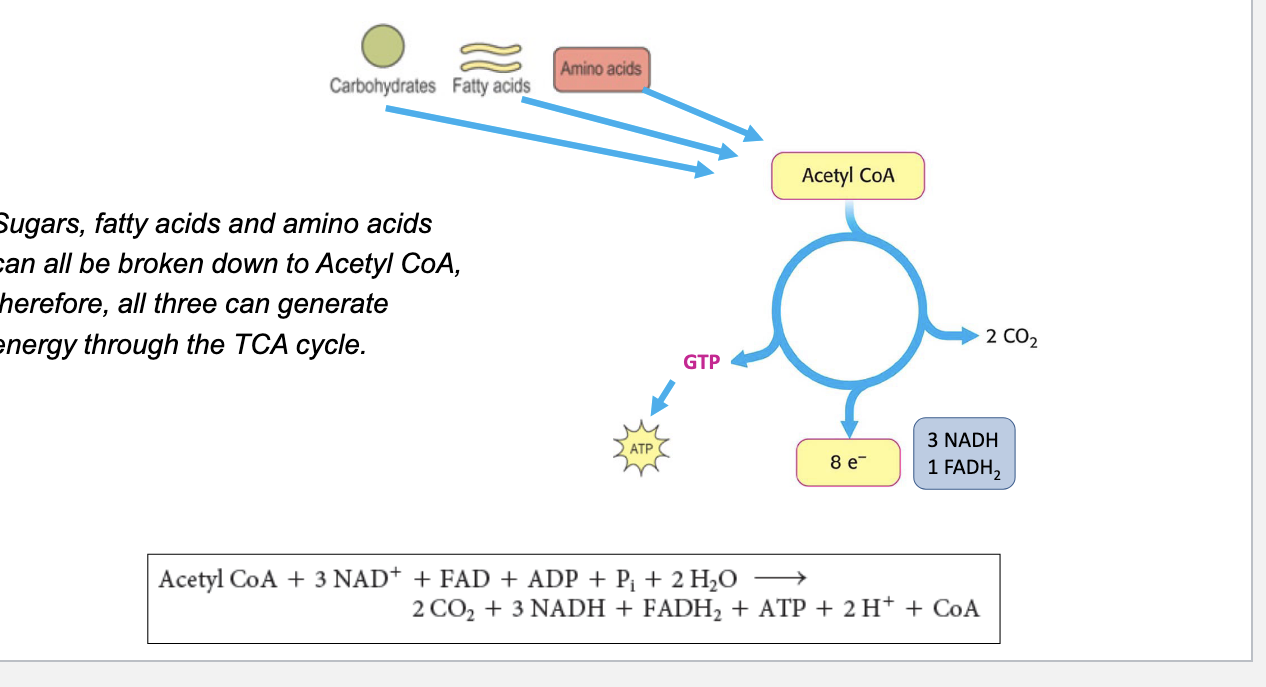
breakdown of carbs, AA, and fatty acids
sugars, fatty acids and AA can all be broken down to acetyl coA → all 3 can generate energy through the TCA cycle
final reaction
Acetyl CoA + 3NAD+ + FAD + ADP + Pi + 2 H2O → 2 CO2 + 3 NADH + FADH2 + ATP + 2H+ + CoA