chapter 6 the proton transfer reaction introduction to mechanisms , charge stability, equilibria, and free energy diagrams
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
Q: What does a low pKa indicate?
A: A strong acid (high tendency to donate a proton).
Q: What does a high pKa indicate?
A: A weak acid (low tendency to donate a proton).
Q: How does acid strength relate to conjugate base strength?
A: Strong acids have weak conjugate bases; weak acids have strong conjugate bases.
Q: Rank these acids by increasing strength: H₂O, HCl, HF.
A: H₂O < HF < HCl
Q: How does electronegativity affect acid strength?
A: More electronegative atoms stabilize the conjugate base, making the acid stronger.
Q: Why should the solvent not react with reactants in a reaction?
A: So the reactants can react as intended; solvent reaction can cause undesired proton transfer.
Q: What happens if a reactant is too strong in a solvent?
A: It can react with the solvent instead of the intended reaction, causing undesired proton transfer.
Q: What is the leveling effect?
A: The phenomenon where the strongest acid or base in a solution is limited by the solvent; stronger acids/bases react with the solvent.
Q: In the leveling effect, what is the strongest acid that can exist in solution?
A: The protonated solvent (e.g., H₃O⁺ in water).
Q: In the leveling effect, what is the strongest base that can exist in solution?
A: The deprotonated solvent (e.g., OH⁻ in water).
Q: Give an example of the leveling effect with HCl in water.
A: HCl is stronger than H₃O⁺, so it donates its proton to water forming H₃O⁺; HCl can’t exist stronger than H₃O⁺ in water.
Q: How does the choice of solvent affect acid-base reactions?
A: Strong acids/bases may be “leveled” by the solvent; choosing a less reactive solvent allows stronger acids/bases to exist.
give the Keq formula
k eq formula example
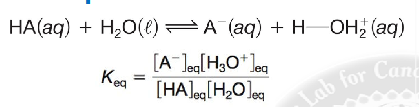
how to convert from Ka to pKa
pKa=-log Ka
*A larger value of
Ka , corresponds to a lower
value of p
Ka.
A lower p
K a value represents a stronger acid.
When two acids are compared, the one with the
larger
K a value is the stronger acid.
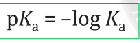
Relative Strengths of Charged and Uncharged Acids:
The Reactivity of Charged Species
A proton is dramatically more acidic when it is attached to a positively charged
atom than when that atom is uncharged.
• The p
K a of H 3O + is 0, whereas that of H2 O is 14.
• The p
K a of H 4N + is 9.4, whereas that of H 3N is 36
Protons on Different Atoms in the Same Column of the Periodic Table
The farther down a column an atom appears in the periodic table, the more
acidic the protons that are attached to it.
p
Ka: HF (3.2) > HCl (−7)
• The negative charge is less densely distributed over larger atoms, which are
found as you go down the column.
• The stability of an anion tends to increase when the negative charge is on
an atom farther down a column of the periodic table.
electronegativity depends on the hybridization of an atom and increases in which order?
sp³< Sp2, < sp
Q: Why is ethanoic acid a stronger acid than ethanol?
A: Its conjugate base (acetate) is resonance stabilized, while ethanol’s conjugate base has a localized charge.
Q: How does resonance affect conjugate base stability?
A: Resonance delocalization stabilizes the negative charge, increasing acid strength.
Q: Ethanol and ethanoic acid are both uncharged acids. Which is stronger and why?
A: Ethanoic acid, because its conjugate base is stabilized by resonance.
Q: General rule: how do resonance effects influence acid strength?
A: Acids whose conjugate bases are resonance-stabilized are stronger than those with localized charges.
stabliztion via resonance generally does what?
generally increases as the number of atoms over which a charge is delocalized increases.
effects from nearby atoms: inductive effects
the presence of nearby electronegative atoms increases the stability of the conjugate base
inductive effect summary
nds on the atom’s electronegativity.
2. Charged substituents have more pronounced inductive effects than
uncharged substituents.
3. Inductive effects are additive.
4. Inductive effects fall off very quickly with distance.
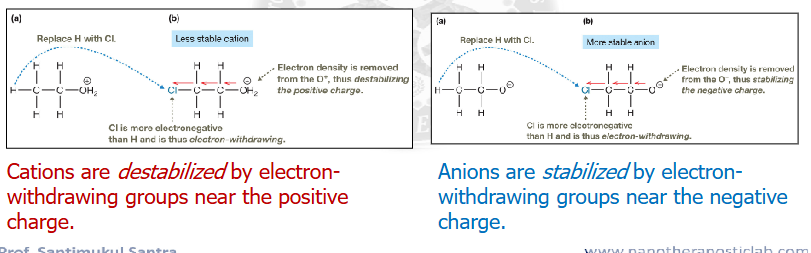
Alkyl groups are electron _______. what are the effects of this
donating.
alkyl groups destabilize anions
and
alkyl groups stabilize cations
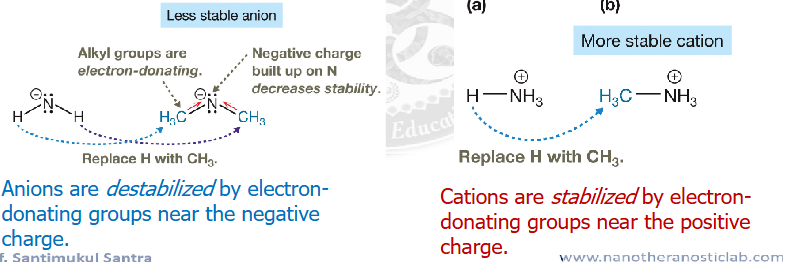
ranking acid and base strengths using “CARDIN-al” RULE
Charge>Atom>resonance delocalization>inductive effects
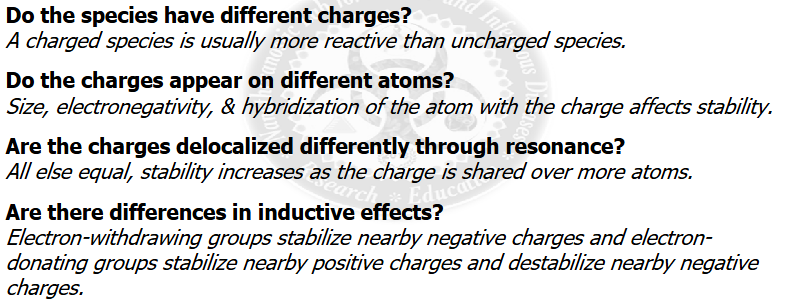
Cardin-al rule summary ranking acid and base strengths
see image about cardin-al rule
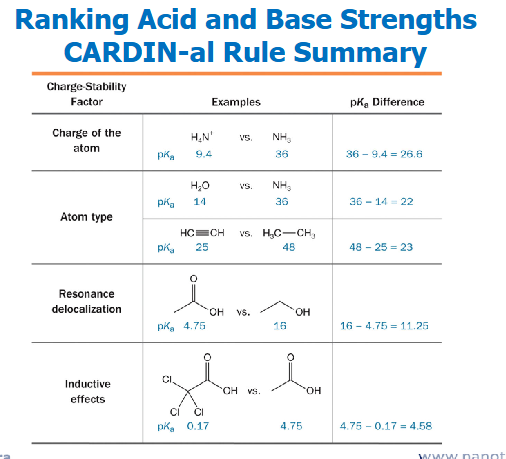
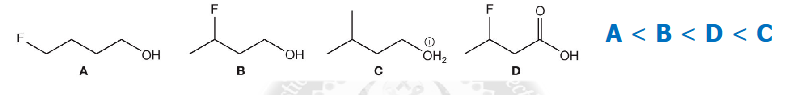
Typical problem: Ranking Acid and Base Strengths
Apply these rules to rank these in terms of increasing acidity (decreasing pKa )
If you know the order of acid strengths, then it is straightforward to determine the order of base strengths
Q: What is heterolysis of a bond?
A: Bond breaking where both electrons go to the same atom.
Q: What is formed when carbon loses both bonding electrons in heterolysis?
A: A carbocation (C⁺).
Q: What is formed when carbon gains both bonding electrons in heterolysis?
A: A carbanion (C⁻).
Q: Example of carbocation formation via heterolysis?
R−Cl→R^+ + Cl^−.
Q: Example of carbanion formation via heterolysis?
R-LI → R^- + Li^=
Q: Why does heterolysis normally require bond polarization?
A: Polarization (due to electronegativity differences) makes it easier for electrons to move to one atom.
what are electrophiles
electron seeeking reagents to achive a stable shell of electrons, like that of a noble gas
ALL LEWIS ACIDS ARE ELECTROPHILES
what are nucleophiles
A lewis base that seeks a positive center such as a positive center such as positively charged carbon atom