(Unit 3) Chapter 8 - An Introduction to Metabolism
1/75
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
76 Terms
Metabolism
Totality of an organism’s chemical reactions (interactions between molecules).
Metabolic Rate
Total amount of energy an animal uses in a unit of time
Relation between organism size and metabolic rate.
Smaller the organism, the higher the metabolic rate.
What is the metabolism’s job?
To manage materials and energy resources of a cell.
Metabolic Pathways and the two types.
A specific molecule is alternated in a series of defined steps, resulting in a certain product.
Catabolic Pathways (Breakdown Pathways): Breaks down complex molecules to simpler compounds to release energy,
Anabolic Pathways (Biosynthetic Pathways): Consume energy to build complex molecules.
Energy
The capacity to cause change
Types of energy
Kinetic: Energy from motion.
Thermal Energy: Movement of atoms or molecules.
Heat: Thermal energy transferred from one object to another.
Light Energy
Potential Energy: Energy that matter possesses because of its location or structure.
Chemical: Energy potential energy available for release in chemical reactions
Energy can be converted from one form to another.
Thermodynamics
The study of the energy transformations that occur in a collection of matter.
Two types of systems
Isolated (Closed) System: Unable to exchange either energy or matter with its surroundings.
Open System: Energy and matter can transferred between the system and its surroundings. Organism fall under this.
First Law of Thermodynamics
Principle of conversation of energy, the energy of the universe is constant. The energy can be transferred or transformed, but it cannot be created or destroyed.
Second Law of Thermodynamics
During every energy or transformation, some energy is converted to thermal energy and released as heat, becoming unavailable to dowkr. Every energy transfer or transformation increases the entropy of the universe.
Thermoregulation
Maintain an internal temperature within a tolerable range.
How do animals maintain thermoregulation?
Endothermic: Thermal energy generated by metabolism to maintain homeostatic body temperatures (birds and mammals). Temperature regulator, internal heat stays the same.
Ectothermic: Gain heat from external sources (invertebrates, fishes, amphibians, and non-avian reptiles). Temperature Conformer, internal heat changes depending on environment.
When can thermal energy be put to work?
When there is a temperature difference. (Thermal energy flowing as heat from warmer to cool).
What makes the universe more disorded?
Released thermal energu
Disorder
How dispersed the energy is in a system and how many different energy levels are present.
Entropy
Measure of molecular disorder (randomness).
Spontaneous Process
Spontaneous: A process that leads to an increase in entropy, requires no energy (energetically favorable
Non-spontaneous Process
A process that leads to a decrease in entropy, requires energy. (Not energetically favorable.)
How do organisms create ordered structures?
They take in organized forms of matter and energy from the surroundings and replace them with less ordered forms.
The entropy of the system decreases, the entropy of the universe increase.
Free Energy
The portion of a system’s energy that can perform work when temperature and pressure are uniform throughout the system.
A measure of a system’s instability, its tendency to change to a more stable state.
Free Energy Equation
ΔG = ΔH - TΔS
ΔG: Change in free energy.
ΔH: Change in system’s enthalpy (total energy)
ΔS: Change in system’s entropy
T: Absolute temperature (K units)
Free Energy Equation (Negative & Positive ΔG)
Negative ΔG is spontaneous.
ΔH is negative as the system gives up energy
OR
TΔS must be positive the system gives up order
OR
ΔH and TΔS are tallied
Every spontaneous process decreases the system’s free energy.
Positive/Zero ΔG is non spontaneous
What does a decrease in free energy mean?
The final state is less likely to change and is more stable.
Equilibrium
Forward and reverse reactions occur at the same rate, no net change in concentration of products and reactants. (Maximum stability, lowest G).
What does moving away from equilibrium mean?
Non spontaneous, increasing free energy.
Two types of reactions in metabolism
Exergonic reactions (“energy outward”) proceeds with a net release of free energy.
Free energy is released to do work.
Endergonic reactions (“energy inward”) absorbs free energy from its surroundings.
Are organisms at equilibrium?
No, systems at equilibrium are at minimum G and can do no work, it has died.
How do cells release energy?
In a series of reactions, some reversible reactions are constantly pulled in one direction to avoid equilibrium.
The product of a reaction does not accumulate but instead becomes a reactant in the next step.
Waste products are expelled from the cell.
Three types of work cells do.
Chemical: Pushing of endergonic reactions.
Transport: The pumping of substances across membranes.
Mechanical: Beating of cilia, contraction of muscle cells, and the movement of chromosomes during cellular reproduction.
Energy coupling
The use of an exergonic process to drive an endergonic one. (ATP)
Structure of ATP
(Adenosine Triphosphate) Sugar ribosome, with nitrogenous base adenine and a chain of three phosphate groups bonded to it.
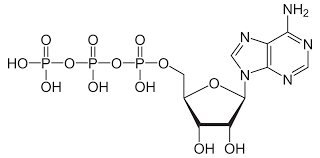
What is ATP used for?
Used in energy coupling and to make RNA.
ATP Hydrolysis
The last phosphate is broken by the addition of a water molecule (ATP becomes ADP).
Why does ATP hydrolysis release so much energy?
The triphosphates of the ATP are all negatively charged they are replushed from eachother and create instability (Compressed spring).
How does ATP provide energy to perform work?
ATP hydrolysis releases heat which can sometimes be useful (shivering) however in most cases it’s inefficient so the cell’s proteins harness the energy and use it do cellular work.
When can reactions be coupled (ATP)?
When the free energy of an endergonic reaction is less than the amount of energy released by ATP hydrolysis.
Phosphorylation
The transfer of a phosphate group from ATP to some other molecule.
Phosphorylated Intermediate
Recipient molecule (ATP hydrolysis) with the phosphate group covalently bonded to them.
What cell work is ATP hydrolysis used in?
Transport and mechanical.
This leads to a change in protein’s shape and it’s ability to bind to another molecule.
Example: Motor proteins, as ATP binds and hydrolyzes the protein changes shape allowing it to bind to the cytoskeleton differently and “walk”.
Enzyme
Biological Catalyst, macromolecule that is a chemical agent that speeds up a reaction without being consumed by the reaction.
Why are enzymes important?
So pathways of metabolism do not become congested.
What needs to happen before a reaction?
The reactant molecule needs to be turned into a highly unstable state.
Reactant molecules must absorb energy from their surroundings.
Activation Energy / Free energy of activation (EA)
Initial investment of energy for starting a reaction.
Absorption of thermal energy accelerates the reactant molecules, causing more collision and force and making breakage of bonds more likely.
Transition State
Molecules have absorbed enough energy for bonds to break, unstable condition.
Why can we not increase heat to speed up reactions?
Denatures proteins and kills cells, speeds up all reactions and not just those that are needed.
How does an enzyme speed up reactions?
The enzyme lowers the activation energy barrier. Free energy is not affected.
Substrate
The reactant an enzyme acts on.
Enzymes are very specific with their substrates.
What accounts for enzyme specifically / molecular recongnition?
Protein, unique 3-D configurations.
Active Site
Pocket or groove on the surface of the enzyme where catalysis occurs / where subtrate binds to.
Structure of enzyme
Active site is formed by specific amino acids, the rest of the protein molecules provide a framework that determines the shape of the active site.
Enzyme fluidity
Can take on subtly different shapes and differences in free energy. Active site can change shape.
Induced Fit.
The enzyme changes shape slightly due to weak bonds between the enzyme and substrate causing the active site to enfold the substrate and hold it in place.
Catabolism & Anabolism
Catabolism: Breaking of macromolecules using enzymes.
Anabolism: Forming of macromolecules using enzymes.
Mechanism enzymes use that lower activation energy and speed up a reaction.
In reactions with multiple reactants, the active site provides a template which helps the substrates come together in the proper orientation.
Enzymes stretch substrate molecules toward their transition state form, this breaks chemical bonds,
Provides a better microenvironment.
Amino acids in the active site may participate in the chemical reaction as brief convergent bonding between substrate and side chain of an amino acid may occur.
What affects speed of enzyme?
The initial concentration of substrate. Higher substrate concentration = more substrate molecules available to be catalyzed = increased rate.
Saturated: Concentration of substrate reaches its limit, all enzyme molecules has their active sites engaged.
What is the efficiency of the enzyme affected by?
Environmental factors (temperature and pH) and chemicals.
How does temperature affect enzymes?
Rate of reaction increases with increasing temperature because substrates collide with active sites more. However there is a limit where the enzymatic reaction drops and denatures the protein.
How does pH affect enzymes?
Too acidic or basic can denature proteins by disrupting H-bonds.
Cofactors
Nonprotein helps for catalytic activity. (Chemical processes like electron transfers) Ex: Zn, Fe, Cu
How do cofactors work?
By binding tightly to the enzyme as permanent residents or bound loosely.
Coenzyme
Cofactors that are organic (vitamins).
When is an inhibitor irreversible/reversible?
Irreversible: Covalent bonds. (Toxins and poisons)
Reversible: Weak Interactions
Competitive Inhibitors
Resemble normal substrate molecules and compete for admission into active site.
Reduce productivity of enzyme by blocking substrates from entering active sites.
Can be over come by increasing concentration of substrate.
Noncompetitive Inhibitors
Impede enzymatic reactions by binding to another part of the enzyme.
causes the enzyme to change its shape leading to a less effective active site.
How do cells regulate metabolic pathways?
By controlling when and where various enzymes are active, switching off and on genes that encode specific enzymes.
How are is enzyme activity regulated?
Molecules behave like reversible noncompetitive inhibitors, switching genes on and off.
How are enzymes that are allosterically regulated constructed?
Two or more subunits with its own active sites.
Allosteric Regualtion
Protein’s function at one site if affected by binding of a regulatory molecule to a separate site (allosteric site).
Two types of allosteric regulation
Activator: Stabilizes the shape that has functional active shape.
Inhibitor: Stabilize the inactive form of the enzyme.
Where do molecules bind in allosteric regulation?
Activating/Inhibiting regulatory molecules bind to a regulatory site (allosteric site), often located where subunits join.
Cooperativity
A substrate molecule binds to one active sites more triggering a shape change in all subunits, increasing catalytic activity at the other active sites.
Feedback Inhibition
A metabolic pathway is halted by the inhibitory binding of its end product to an enzyme that acts early in the pathway.
Prevents cells from making more of a certain product than necessary and wasting chemical resources, increased efficiency.
How does feedback inhibition work?
The end product of a metabolic pathway shuts down pathway by binding to the allosteric site of an enzyme.
Allosteric Site
A specific location on a protein or enzyme that is distinct from the active sites more, where binding molecules can alter the protein’s shape and function.
Localization of enzymes within the cell
A team of enzymes for several steps are assembled into a multi enzyme complex, facilitating the sequence of reactions.