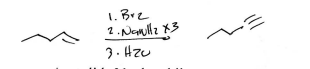OCHEM 2 Synthesis Reagents
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
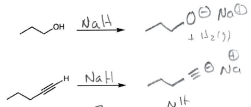
NaH
Creates a good leaving group
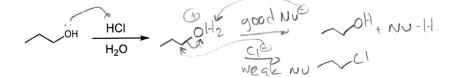
HCl + H2O
Alcohol to good leaving group
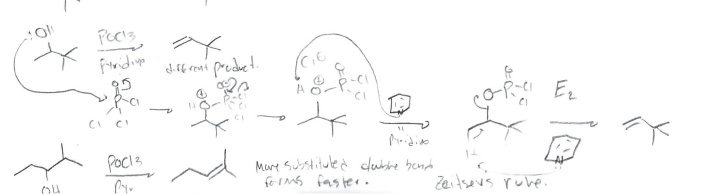
POCl3 + Pyridine
Elimination at most substituted carbon near alcohol (Zaitzev’s Rule)
H2SO4
Elimination, forms double bond at most substituted carbon in molecule due to carbocation (+) formation
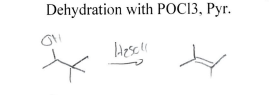
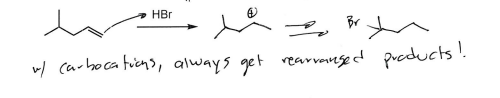
HBr
Alcohol to good leaving group; if alcohol on non-primary Carbon, carbocation rearrangement
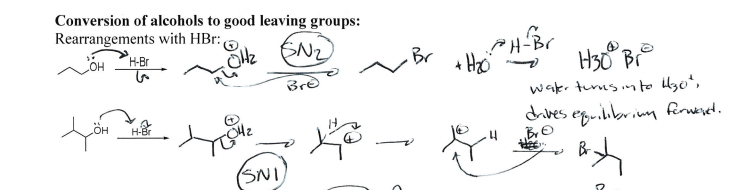
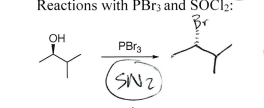
PBr3
Inversion, substitution of good leaving group
SOCl2
Inversion, substitution of good leaving group
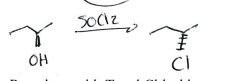
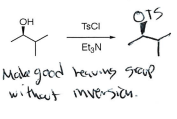
TsCl + Et3N
No inversion, addition of good leaving group (OTs)
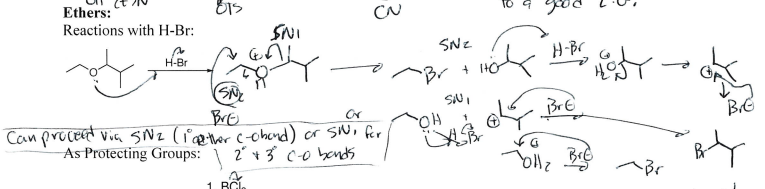
HBr + Ether
Ether cleavage —> Br Addition on larger side + small side alcohol
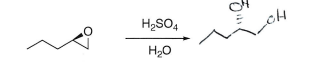
Epoxides (Acid)
Inversion alcohol addition at more sub carbon
Epoxides (Strong Nu-)
Inversion alcohol addition at less sub carbon
H2SO4 + H2O (Alkene)
Alcohol addition at must substituted carbon
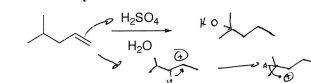
HBr (Alkene)
Br addition at most substituted carbon
HCl + H2O (Alkene)
Alcohol addition at most substituted carbon

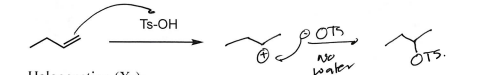
Ts-OH (Alkene)
OTs addition at most substituted carbon
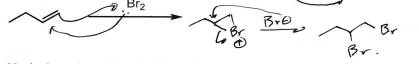
Br2 (Alkene)
Dibromide due to Ketone-like Br initial addition
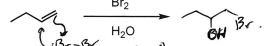
Br2 + H2O (Alkene)
Br addition at least substituted carbon, Alcohol addition at most substituted carbon
BH3 (Alkene)
B + 3 Alkane extension (Anti-Markovnikov)
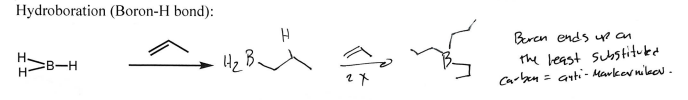
BH3
H2O2 + NaOH
(Alkene)
Alcohol addition at less substituted carbon (Anti-Markovnikov); selectivity = opposite adjacent R group

HBr (Alkyne)
2 Br at most substituted carbon
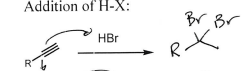
H2SO4 + HgSO4 + H2O (Alkyne)
Ketone at most substituted Carbon
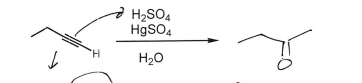
BH3
H2O2, NaOH
(Alkyne)
Ketone at least substituted carbon (Anti-Markovnikov)
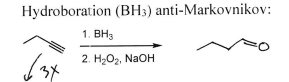
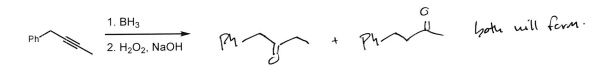
Ph Group 1. BH3 2. H2O2 + NaOH
Ketone at must substituted carbons (multiple products)
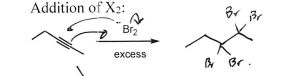
Br2 (Alkyne)
Dibromide at each triple bond terminus
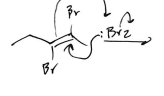
Br2 Intermediate (Alkyne)
Single Br addition at each triple bond terminus
NaNH2 × 3
Alkyne Synthesis (Strong Nucleophile)
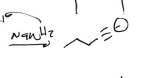
Br2
NaNH2 × 3
H2O
(Alkene)
Alkyne with H end synthesis