Unit 2: Micro and Macro Molecules
1/136
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
137 Terms
ionic bond
tend to be between metals and non-metals
one element (anion) takes e- and the other (cation) loses e-
kept together by the electrostatic attraction in solid phase
separates into ions when in water, forms electrolytes, and conducts electricity
covalent bond
Tend to be between non-metals
Both share electrons, neither forms an ion
Kept together by shared electrons that give each atom eight valence electrons
Does not generally separate in water, make poor electrolytes, and do not conduct electricity
How is the polarity of intramolecular bonds notated?
by 𝛿+ and 𝛿- or arrows with a plus (⇸)
electrostatic force
the attraction of opposite charges (+/-)
overall dipole
molecules that are polar overall
O-H bond
polar
N-H bond
polar
C-H bond
non-polar
asymmetrical molecule
usually polar
symmetrical molecule
usually non-polar
hydrogen bonds
water molecules are interconnected with electrostatic force due to high polarity
notated by dashed lines
not an actual chemical bond
intermolecular force (IMF)
attractions between molecules
intramolecular force
bonding between atoms to make a molecule (covalent, ionic)
electronegativity (EN)
the ability of an atom to draw electrons towards itself in a chemical bond
(0-0.4) non-polar covalent: electrons shared equally
(0.4-1.8) polar covalent: electrons not shared equally; higher EN is pulling towards itself
(1.8-4.0+) ionic: electron is fully stripped from lower EN to higher EN
Which elements have the highest EN?
Fluorine, Oxygen, Nitrogen
solubility
the ability to be dissolved in a solvent
solvent
the dissolving agent of a solution
solute
the substance that is dissolved
aqueous solution
a solution in which the solvent is water
hydrophobic
does not dissolve in water
hydrophilic
dissolves in water
electrolyte
an ionic compound that conducts electricity in water by splitting up into separate ions
How does a solute dissolve in water?
The polar sides of water molecules attract their opposites with electrostatic force and dissolve polar covalent/ionic bonds. Ionic bonds are separated fully into cations and anions (electrolytes).
What does solubility depend on?
Size and polarity; adding more non-polar regions to a molecule will decrease its solubility in water
specific heat capacity
the amount of energy required to change a substance’s temperature
How does the specific heat capacity of water work?
Water takes a long time to boil, and it boils at a high temperature; since the hydrogen bonds are so strong, you need to add more heat to separate the bonds (to give them more heat/energy) to change them into the gas state
evaporative cooling
the heat of your body separates and evaporates the water (sweat)
adhesion
the attractive force between water molecules and other molecules ex. the sides of a beaker are more polar than water, attracting the water molecules to the sides of the beaker; makes a concave meniscus
cohesion
the attractive force between water molecules and themselves ex. surface tension
Why don’t oil and water mix?
Oil and water do not mix since oil is not polar/not attracted to water molecules and cannot break through the hydrogen bonds; the layering facilitates from the density of the liquids, where since water’s hydrogen bonds are very tight, there have a higher density than oil, causing the formation of layers.
acid base reaction
H+(aq) + OH-(aq) = H2O(1)
acidic
more H+ ions, lower pH
basic
more OH- ions, higher pH
neutral
H+ ions equal OH- ions, pH of 7
acid
A substance that adds hydrogen ions (H+) to solutions ex. hydrochloric acid
base
a substance that reduces the hydrogen ion concentration of a solution, by either increasing the OH- concentration or by removing H+ ex. sodium hydroxide base, bicarbonate base
sodium hydroxide base reaction
NaOH(aq)=Na+(aq)+OH-(aq)
bicarbonate base reaction
HCO-3(aq)+H+(aq)=H2CO3(aq)
equilibrium
the balance between the product and reactant (⇄)
pH scale
measures the concentration of H+, thereby describing the acidity or alkalinity (basicity)
lower pH means more acidic, higher pH means more basic, 7 is neutral
the concentration of H+ decreases by 10x every pH level jump
weak acid
doesn’t separate very much (~5%)
strong acid
completely dissociated (~100%)
buffer
resists change in pH; a special mixture of a weak acid and a conjugate base
conjugate pair
two molecules containing the same negative ion where the base has one less carbon ex. H2CO3 (carbonic acid), HCO3- (bicarbonate)
increase in blood pH
H+ is removed from the bloodstream.
Carbonic acid (H2CO3) is used up by the body to release H+ and bicarbonate (HCO3-) into the bloodstream.
Since carbonic acid is being used up, the body needs more water and CO2 to replenish the used carbonic acid.
The respiration rate decreases to hold onto extra CO2.
decrease in blood pH
H+ is added to the bloodstream.
Carbonic acid (H2CO3) is made by those H+ reacting with bicarbonate (HCO3-) that is stored in the body in reserve.
Carbonic acid breaks down into CO2 and water.
The respiration rate increases to release the extra CO2.
abiogenesis
the idea that life came from non-living chemistry
biogenesis
the idea that life comes from life
Miller-Urey experiment
an experiment that simulates the ancient atmosphere and water cycle to test if monomers (ex. amino acids) can form from basic molecules; supported and found amino acids
spontaneous generation
theory that life is continuously coming from nonliving sources (disproved by Pasteur ex. steak/flies)
Macromolecules
large molecules important in the chemistry of life
organic compounds
carbon-based molecules
Hydrocarbons
compounds made of hydrogen and carbon (non-polar)
How many bonds will hydrogen, oxygen, carbon, and nitrogen have?
1, 2, 4, and 3/4 (can be double bonds)
isomers
molecules that have the same formula but different structures
constitutional (structural) isomers
different bonds (ex. glucose/fructose)
stereoisomers (spatial isomers)
same bonds, different organization
enantiomers
mirror images of each other (stereoisomer)
geometric isomers
different about their double bond (stereoisomer)
cis/trans isomers
require a double bond to lock into place
cis
bonds are on the same side of the double bond
trans
bonds are on opposite sides of the double bond
monomer
a small organic particle
dimer
two monomers connected through dehydration synthesis
polymer
a macromolecule composed of repeating monomers
polymerization
the process of making polymers from monomers using enzymes
dehydration synthesis
a polymer with OH- bonds with the H+ from a monomer, where the water molecule is released and the bond is held with a leftover atom(s)
hydrolysis
a reaction when water is added to a polymer, breaking the bonds between them creating monomers
structural formula
every single atom and bond is shown in the drawing
abbreviated structure
internal carbon atoms are replaced by lines/vertices
simplified structure
most carbon atoms are implied, recognized by shape
functional group
groups added to molecules that change the properties of that molecule
hydroxyl group
OH, polar, hydrophilic, hydrogen bonding site
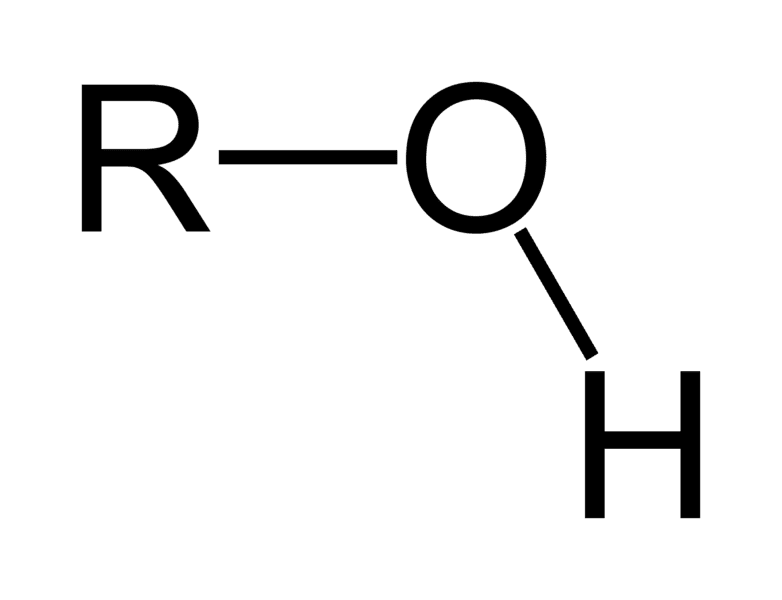
carbonyl group
CO, somewhat polar, increases water solubility

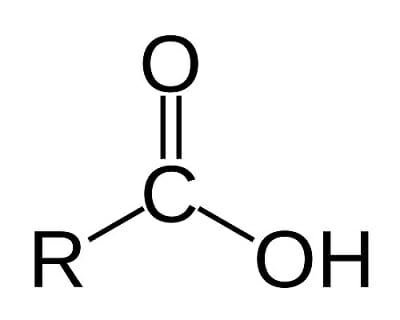
carboxyl group
COOH, polar, hydrophilic, hydrogen bonding site, becomes negatively ionic/acidic by releasing the hydrogen
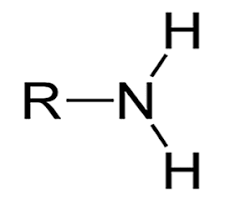
amine group
NH2, polar, hydrophilic, becomes positively ionic/basic by taking in hydrogen
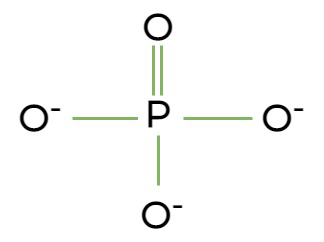
phosphate group
OPO32-, polar, hydrophilic, can take ionic charge
methyl group
CH3, non-polar, hydrophilic, decreases solubility (comes off the side of a molecule chain or ring, not the end)
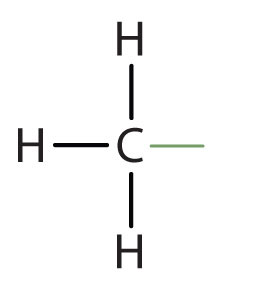
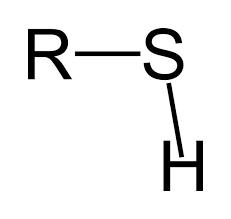
sulfhydryl group
SH, somewhat polar, somewhat hydrophilic, creates disulfide bonds
carbohydrates
monosaccharides, polysaccharides, simple sugars; mixes well with water
monosaccharide
a monomer that typically has a hydroxyl group and a carbonyl group, forms polysaccharides (ex. glucose/fructose
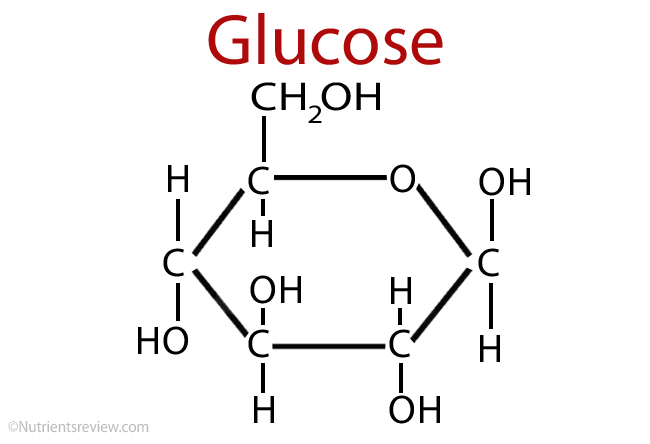
glucose (C6H12O6)
a monosaccharide that can come in linear (power) or ring (water) form
disaccharides
two monosaccharides connected through dehydration synthesis
sucrose
a disaccharide made of glucose and fructose
lactose
a disaccharide made of galactose and glucose
maltose
a disaccharide made of glucose and glucose
glycosidic bonds
holds two monosaccharides together
polysaccharides
made of hundreds to thousands of monosaccharides and glycosidic bonds
starch
a polysaccharide made of one strand of glucose; used by plants to story energy
glycogen
a polysaccharide made of branches of glucose; stored in animal liver and muscle cells
cellulose
a polysaccharide made of parallel glucose strands connected by hydrogen bonds; most abundant organic compound; animals do not have the enzymes to digest them (fiber)
chitin
similar to cellulose but contains an amine group; found in shells of crustaceans and in some fungi
lipids
diverse group of molecule sharing one trait: have significant hydrophobic regions
triglycerides (fats/oils)
made of a glycerol and 3 fatty acids
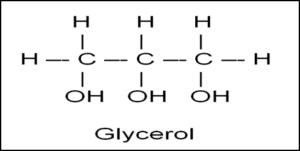
glycerol
a molecule containing hydroxyl groups used in triglycerides and phospholipids
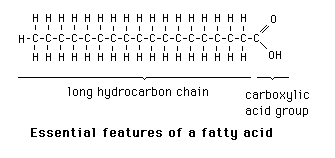
fatty acid
a chain of CH2 with a carboxyl group that reacts with glycerol making ester bonds
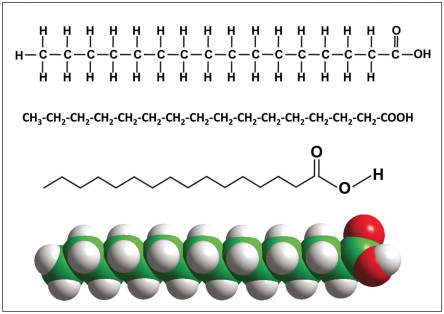
ester bonds
bonds between a fatty acid and glycerol
saturated
no double bonds with the most amount of hydrogen possible, straight line (fat ex. butter)
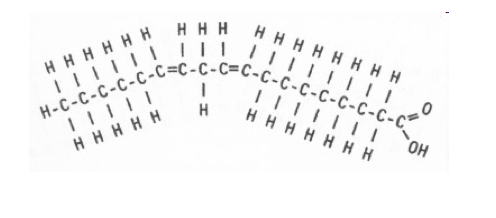
unsaturated
the carbon chain contains 1+ double bond causing bends (oil)
phospholipids
a glycerol and two fatty acids connected with ester bonds; the glycerol has one phosphate group and this molecule is used for cell walls