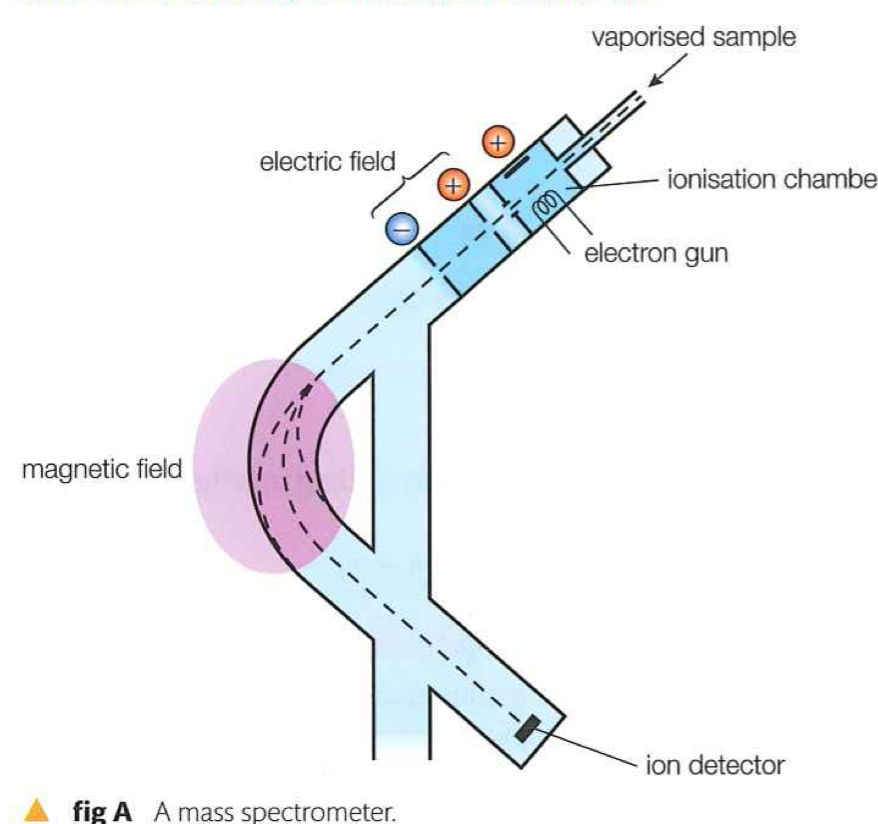
Mass spectrometer
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What does a mass spectrometer do?
Measures masses of atoms and molecules
Produces positive ions that are deflected in magnetic field according to mass to charge ratio
Calculates relative abundances of each positive ions
Step 1 in mass spectrometer
Heater vaporizes the sample so that particles can move through the machine
Step 2 in mass spectrometer
Vapor is bombarded with high energy electrons, resulting in one or more electrons being removed from the atoms, to form positive ions.
Step 3 in mass spectrometer
Electric field causes positive ions to accelerate
Step 5 in mass spectrometer
Positive ions deflect in the uniform magnetic field. The amount of deflection depends on the mass to charge ratio
Diatomic molecules and mass spectrometer
Diatomic molecules can be broken down into single atoms which are detected.
Diatomic molecules’ abundances are based on the abundances of combination of single atoms
Polyatomic molecule and molecular ion peak
There is a small number of C-13 isotopes which can lead to M+1 peak (only in very large molecules)
Why is mass spectrometer operated under a vacuum
So the only particles being detected are those in the sample, none from air.
How does a mass spectrometer help differentiate between isotopes?
By deflecting positive ions and separating them due to their mass to charge ratio.
Mass spectrometer labeled