irreversible inhibitors
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
how effect enzyme
i form unbreakable covalent bond with e, basically kill the e and permanently inactivate it by changing the active side
not all of them use covalent bonds, some use a lot of noncovalent bonds so the sbustr binding is impossible
acetylcholine
acetylcholine is a neurotransmitter at neuromuscular joins and activates the muscles to contract
regulates unconscious functions like hear rate, digestion, respiratory rate, pupillary response, and excretion
acetylcholinesterase
breaks down acetylcholine quickly to allow for further neurotransmission
ser protease (nu: ser, base: his, acid: glu)
inhibiting acetylcholinesterase
inhibitors include: some snake venom, organophosphates like dipf (nerve gas)
results in death bc acetylcholine isn’t being broken down and it overstimulates the receptors
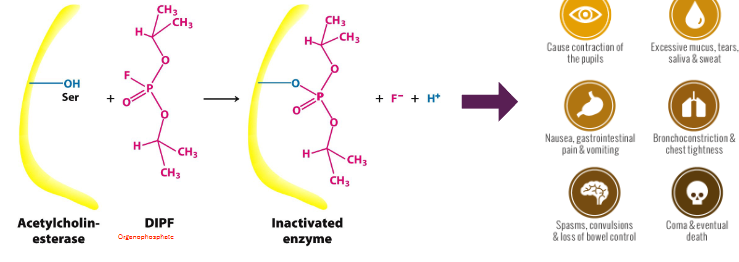
dipf
the p covalently bonds to the o in ser, releasing F- and H+ → can’t be hydrolyzed or broken down so the dipf molec is stuck there and stops the enzyme from working
so deadly bc requires very low dose to kill (under 10mg)
causes pupil contration, excessive mucus, nausea, pain, vomiting, chest tighening, convulsions, etc
e mech and drug design
hiv-1 proteases like to cleave btwn aro residue (tyr or phe) and a pro aa, so we can create a substr that mimics that and binds tight enough to permanently disable the enzyme
transition state analogs
substr that mimic the transition state of a specific enzyme’s substr, max the noncovalent interaxns btwn the drug and active site tighter than the regular substr to prevent any more rxns + they’re more chemically stable
in hiv-1, the oh group of a drug mimics the tetrahedral intmd
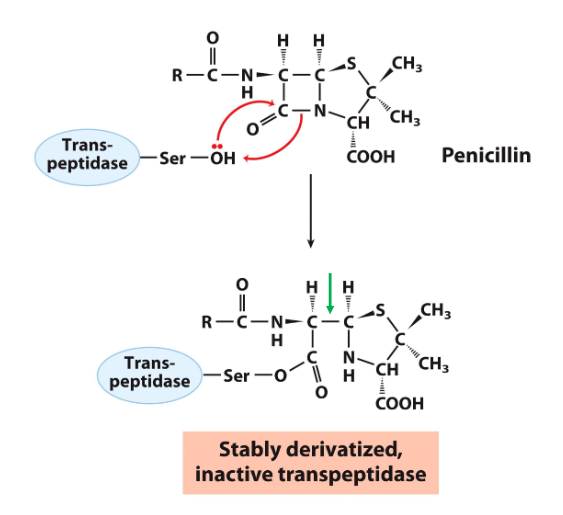
transpeptidatse
transpeptidase is a bact e that makes the cell wall (peptidoglycan) and inhibition of the e or pdction of weak wall causes the bact to die / explode bc of osmotic pressure
similar to ser protease but the 2nd Nu is another peptidoglycan chain so it makes cross hatch pattern
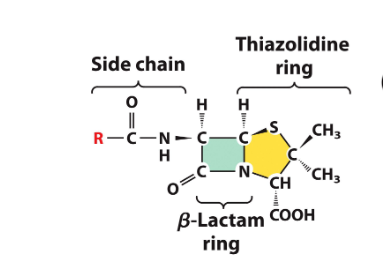
penicillin
excreted by fungi and acts as a transpeptidase inhibitor, killing bact
penicillin as inhibitor
when the carbonyl c is attacked by the e nu, it breaks the bond btwn the carbonyl c and n but bc of the other c-c bond in the beta-lactam ring, the molec isn’t fully separated and nothing leaves
results in the e covalently bound the penicillin and no room for the next nu to come in so the e stops working
how do we get pinicillin resistance
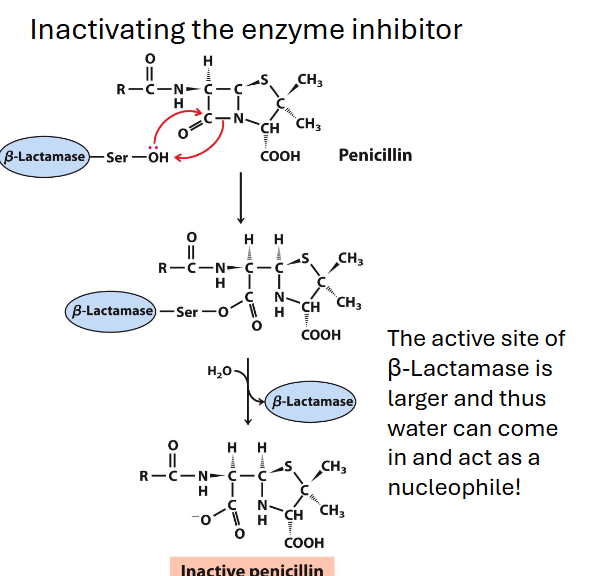
big evo pressure, if you get penicillin close then you die so
bact evolved 2nd e: beta lactamase that has a bigger active site (can fit penicillin inhibitor AND h2o), results in COO attached to the penicillin, inactivating it
inactivating the inhibitor gives cell more time to make more beta lactamase
how do humans get around the beta lactamase inactivating penicillin
synth a beta lactamase specific inactivator, a molec covalently bound to beta lactamase active site