Pharm tech
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
intranasal delivery - nasal anatomy
Two nostrils, separated by a septum
Nasal vestibule (hairy)
Respiratory region
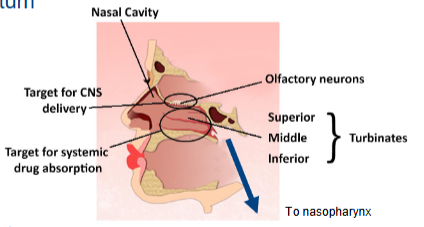
3 turbinates/conchae
Superior, middle, inferior
Olfactory region
Located in the olfactory recess
Direct connection to CNS
Highly vascularized
~15 cm2 (10% of nasal surface
Access to CNS via respiratory & olfactory regions:
Nose to brain access via various permeation routes
intranasal delivery - mode of transport
Transcellular transport
Through active mechanisms through the cell
Slow (upwards of 13 hours)
Paracellular transport
Rapid uptake
Passive transport through gaps between cells
High turnover of olfactory sensory neurons can leave more gaps
Intraneuronal transport
intranasal delivery - advantages
Non-invasive
Can be self-administered
Bypasses the hepatic first-pass effect
Short onset of effect
intranasal delivery - barriers
Nasal epithelial layer
Nasal mucus (~5 μm)
Metabolic enzymes
Efflux pumps
Hair
Mucociliary clearance
Volume
intranasal delivery - ideal drug candidates

intranasal delivery - types of delivery systems

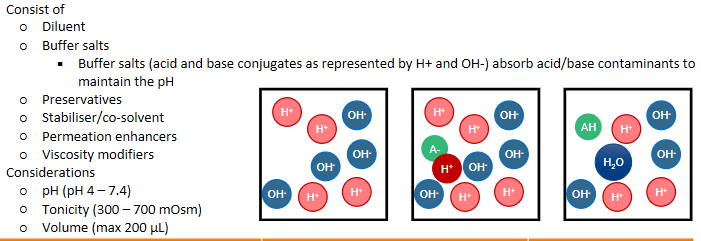
intranasal delivery - Common excipients of nasal sprays
intranasal delivery - Packaging and storing
Container vessel material
Should not have chemical or physical interactions with drug and excipients
Protects the formulation from contamination and degradation
Typically kept in cool and low moisture environments, and not in the fridge/freezer
intranasal delivery - Imitrex/sumatriptan

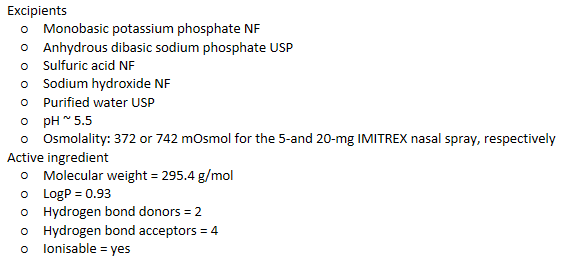
IN Imitrex/sumatriptan - composition
IN Imitrex/sumatriptan - PK
Suspected (but unconfirmed) paracellular transport of the drug. Route for small hydrophilic molecules
60% of peak concentration 30 mins after administration
Second peak after absorption from gut
IN Imitrex/sumatriptan - delivery device (nasal spray)

IN Imitrex/sumatriptan - Unique regulations for nasal sprays
Pump delivery
Durability of device
Reproducibility of pump spray weight
Spray Content Uniformity (SCU)
Amount of drug delivered per pump
Spray pattern and plume geometry
IN Imitrex/sumatriptan - Delivery device (powder)
Mouth nozzle to blow powder through another nozzle inserted in the nostril
Blowing through the mouth avoids negative pressure and "traps" powder in nasal cavity
Less loss of drug
May cause irritation
intranasal delivery - Nayzilam/midazolam
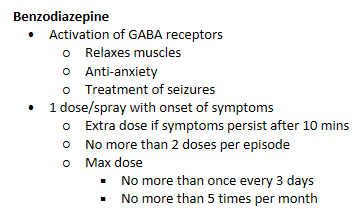
IN Nayzilam/midazolam - midazolam
Molecular weight = 325.77 g/mol
LogP = 3.97
Hydrogen bond donors = 0
Hydrogen bond acceptors = 3
Ionisable: no
IN Nayzilam/midazolam - composition
Ethanol
PEG-6 methyl ether
Polyethylene glycol 400
Propylene glycol
Purified water
Why no buffers/pH adjusting agents?
intranasal delivery - in situ gels
Low viscosity solutions initially but increase in viscosity once administered
Activated by stimulus such as changes in salt concentrations, pH, temperature etc.
Can enhance retention time
No products on market yet
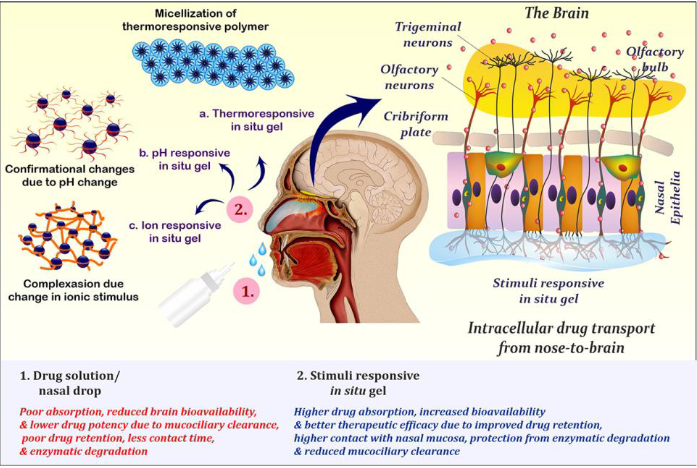
in situ gels - composition
