24.2 ALIPHATIC HYDROCARBONS
0.0(0)
Card Sorting
1/10
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
1
New cards
What are the two types of hydrocarbons?
Aliphatic and Aromatic
2
New cards
What is the general formula of Alkanes?
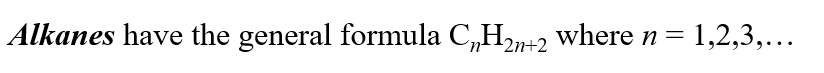
3
New cards
What type of bonds can alkanes form?
single covalent bonds
4
New cards
Why are alkanes saturated hydrocarbons?
they contain the maximum number of hydrogen atoms that can bond with the number of carbon atoms in the molecule
5
New cards
Examples of alkanes
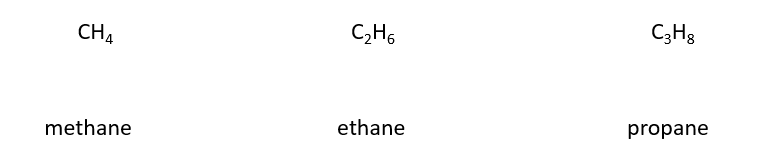
6
New cards
What are isomers?
compounds that have the same molecular formula but different arrangement
7
New cards
8
New cards
What are CH4 and CH3 each called?
CH4 : methane
CH3 : methyl
9
New cards
What are the two types of alkane reactions?
Combustion
Halogenation
10
New cards
What are alkanes in which one or more hydrogen atoms have been replaced by a halogen ?
alkyl halides
11
New cards
What is the general formula of Alkenes?