MT Laws Week 11
1/78
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
79 Terms
Comprehensive Dangerous Drugs Act of 2002
REPUBLIC ACT NO. 9165 shall be known and cited as the “___”
RA 6425
REPUBLIC ACT NO. 9165 repeals ___
500,000 to 10,000,000
Section 11. Possession of Dangerous Drugs
The penalty of life imprisonment to death and a fine ranging from ___ to ___
opium ; morphine ; heroin; cocaine ; marijuana resin or marijuana resin oil
Section 11. Possession of Dangerous Drugs
10 grams of:
___
___
___
___
___ or ___
methamphetamine hydrochloride
Section 11. Possession of Dangerous Drugs
50 grams of:
___
marijuana
Section 11. Possession of Dangerous Drugs
500 grams of:
___
screening test and confirmatory test
Section 36. Authorized Drug Testing.
The drug testing shall employ, among others, two (2) testing methods ___ and ___
one year
Drug test certificates issued by accredited drug testing centers shall be valid for a ___ period from the date of issue which may be used for other purposes
Random Drug Testing
Students and Employees may undergo
six years and one day
All persons charged before the prosecutor's office with a criminal offense having an imposable penalty of imprisonment of not less than ___ and ___ shall have to undergo a mandatory drug test
six years and one day to twelve years
Section 37. Issuance of False or Fraudulent Drug Test Results.
Any person authorized, licensed or accredited under this Act and its implementing rules to conduct drug examination or test, who issues false or fraudulent drug test results knowingly, willfully or through gross negligence, shall suffer the penalty of imprisonment ranging from ___ to ___
100,000 ; 500,000
Section 37. Issuance of False or Fraudulent Drug Test Results.
Any person authorized, licensed or accredited under this Act and its implementing rules to conduct drug examination or test, who issues false or fraudulent drug test results knowingly, willfully or through gross negligence, shall suffer a fine of ___ to ___
revocation of license
Section 37. Issuance of False or Fraudulent Drug Test Results.
Any person authorized, licensed or accredited under this Act and its implementing rules to conduct drug examination or test, who issues false or fraudulent drug test results knowingly, willfully or through gross negligence, shall suffer a ___
closure of the drug testing center
Section 37. Issuance of False or Fraudulent Drug Test Results.
Any person authorized, licensed or accredited under this Act and its implementing rules to conduct drug examination or test, who issues false or fraudulent drug test results knowingly, willfully or through gross negligence, shall suffer the ___
24 hours
Section 38. Laboratory Examination or Test on Apprehended/Arrested Offenders.
Subject to Section 15 of this Act, any person apprehended or arrested for violating the provisions of this Act shall be subjected to screening laboratory examination or test within ___
15 days
If found to be positive, the results of the screening laboratory examination or test shall be challenged within ___
gas chromatograph / mass spectrometry
after receipt of the result through a confirmatory test conducted in any accredited analytical laboratory equipment with a ___ / ___ equipment or some such modern and accepted method
Government Drug Testing Laboratories
Ownership
___ are operated and maintained partially or wholly by the national, provincial, city, or municipal government, or other political unit, or by any department, division, board, or agency thereof.
Private Drug Testing Laboratories
Ownership
___ are privately owned, established, and operated with funds through donation, principal, investment, or other means, by any individual, corporation, association, or organization.
Institution-based Drug Testing Laboratories
Institutional Character
___ are located within the premises and operates as part of an institution (e.g., hospital, medical facilities for overseas workers and seafarers).
Freestanding Drug Testing Laboratories
Institutional Character
___ are located outside the an institution and operate independently.
Screening laboratories
Service Capability
___ are capable of performing screening tests.
Confirmatory laboratories
Service Capability
___ are capable of performing qualitative and quantitative examinations of dangerous drugs from the specimen.
pathologist or licensed physician with clinical laboratory management training
Freestanding screening DTLs must be headed by:
___ or ___
licensed physician ; chemist ; medical technologist ; pharmacist ; chemical engineer
Institution-based screening DTLs must be headed by a
___
___
___
___
___
pathologist or chemist with Master's degree in chemistry or biochemistry.
Confirmatory DTLs must be headed by
___ or ___
Chemist ; Medical Technologist ; Pharmacist ; Chemical engineer
Certified Drug Test Analyst (DTA)
___
___
___
___
10
A licensed physician is allowed to supervise at least _ DTLs
chemists, medical technologists, pharmacists, or chemical engineers
Qualified professionals such ___, ___, ___, or ___ may perform the tasks of a drug analyst
specimen collector
A ___ is a member of the laboratory personnel who collects specimens from a patient, client, or subject.
East Avenue Medical Center
The National Reference Laboratory of toxicology, including drug test is the ___
20 sqm ; 10 sqm
Screening DTL
Floor Area: ___
Work Area: ___
60 sqm ; 30 sqm
Confirmatory DTL
Floor Area: ___
Work Area: ___
time of collection to receipt
These steps require the confirmation of the applicant's identity and a custody and control form (CCF) must be used from the ___ to ___ by the laboratory
60 mL
urination and to provide at least ___ collected in single specimen
30 mL
urination and to provide at least ___ collected in split specimen
laboratory validity testing
A tampered specimen is sent to the ___ and the authorized specimen collector shall document the tampering on the chain of custody form (CCF) with appropriate remarks
temperature ; volume ; physical characteristics
After the client/donor/subject hands the specimen/the authorized specimen collector must measure the ___, check ___, and inspect its ___
tamper-evident label/seal
A ___ must be used to secure the specimen container
authorized specimen collector and subject
Both the ___ and the ___ must affix their signature on the seal together with the date and time of collection.
Screening test (qualitative test)
___ refers to a test to eliminate negative specimens from further consideration and to identify the presumptively positive specimens that requires confirmatory testing.
Confirmatory test (quantitative test)
___ refers to the analytical procedure used to identify and quantify the presence of a specific drug or metabolite, which is independent of the initial test and which uses a different technique and chemical principle from that of the screening test in order to ensure reliability and accuracy.
antibody-antigen reaction
Immunoassay: These methods are used for preliminary screening based on ___.
chromatographic method
Chromatography. The separation of a mixture is the main outcome of the ___. In this process, a mixture of the substance is separated in a stationary medium.
Hyphenated technique
___: Combination of two sophisticated technologies (i.e., gas chromatography - mass spectrometry or GC-MS) or other modern and acceptable techniques (i.e., LC-MS, GC-MS-MS, or LC-MS-MS).
1 year
Screening test Accreditation valid for: ___
2 year
Confirmatory test Accreditation valid for: ___
positive screening test results
All specimens with ___ are submitted for confirmation before a final report will be issued.
analyst and the head of the DTL
All test results obtained either through screening or confirmatory testing shall bear the signature of the ___ and the ___.
one year
All test results obtained either through screening or confirmatory testing are valid for ___ from the date of issue.
90 days
The application for renewal of accreditation shall be filed ___ before the expiry date.
three weeks
The laboratory shall submit to the NRL the results of the test performed on the unknown sample within ___ after the receipt of the test sample
Amphetamines
___: Narcolepsy, Attentional deficit disorder
Methamphetamine Hydrochloride
Amphetamines
Derivative of methamphetamine: ___
Methylenedioxymethamphetamine (MDMA)
Amphetamines
Derivative of amphetamines: ___
Anabolic Steroids
___: Increasing muscle mass
Cannabinoids
___: Hallucinogen and induce euphoria
11-nor-Delta(9)-tetrahydrocannabinol-9-COOH (THC)
Cannabinoids
Urine metabolite: ___
Cocaine
___: Local anesthetic for nasopharyngeal surgery
Benzoylecgonine
Cocaine
Urine metabolite: ___
Opiates
___: Analgesia, Sedation & Anesthesia
N-acetylmorphine & Morphine
Opiates
Major metabolites of Heroin: ___
Phencyclidine
___: Analgesia & Anesthesia
Phencyclidine HCl
Phencyclidine
Major metabolite: ___
Sedative Hypnotics
___: CNS depressants
Secobarbital
Sedative Hypnotics
Major metabolite: ___
Lysergic Acid Diethylamide
___: Hallucinogen
2-oxo-3-hydroxy LSD
Lysergic Acid Diethylamide
Major metabolite: ___
Methaqualone
___: Hallucinogen
Piperazines
___: same with Amphetamines A E.g. TFMPP or Molly, BZP
Tryptamine
___: E.g. DMT (causes Businessman's lunch), Psilocin
Cathinones
___: Psychoactive designer drugs
fifteen days
Specimens with confirmed positive test results, which are not challenged within ___ after receipt, shall be discarded
one year
A specimen may be kept for a maximum of ___ upon request
5 days
A minimum of ___ for negative specimens
Positive
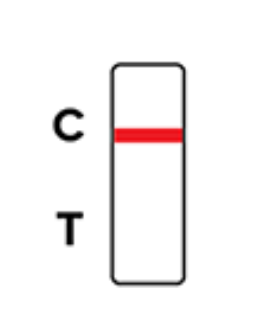
Negative
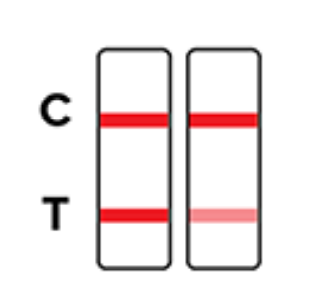
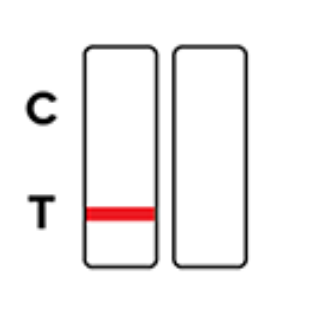
Invalid
Computer
1 IDTOMIS = 1 ___