Intermolecular bonding - Hydrogen bonding
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
What are the three most electronegative atoms?
Nitrogen, oxygen, fluorine
What is hydrogen bonding?
Occurs in compounds that have a hydrogen atom attached to one of the three most electronegative atoms (N, O, and F) which must have an available lone pair of electrons.
Where does hydrogen bonding occur?
In molecules where a hydrogen atom is covalently bonded to fluorine, nitrogen or oxygen.
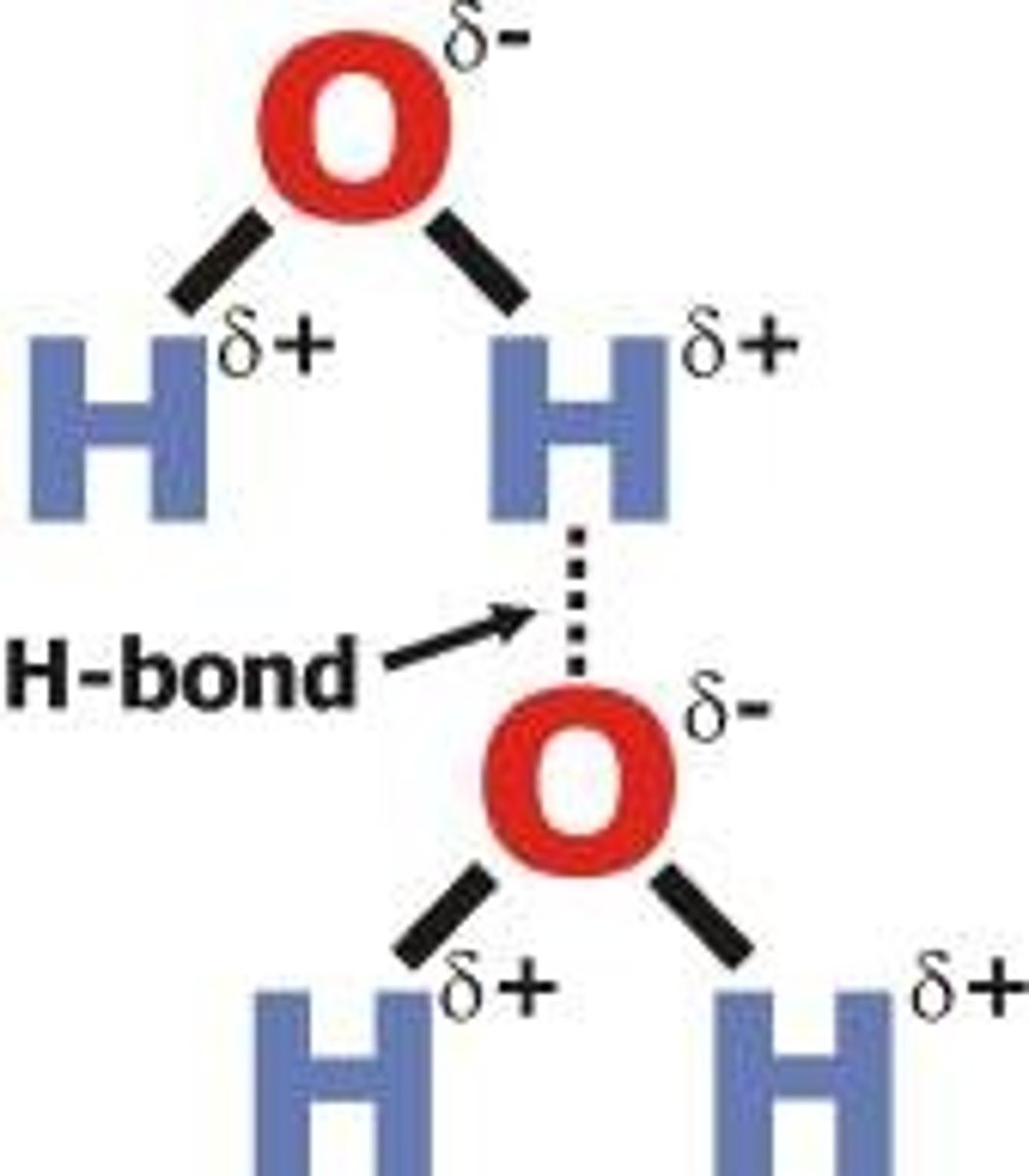
Hydrogen bonding between water molecules
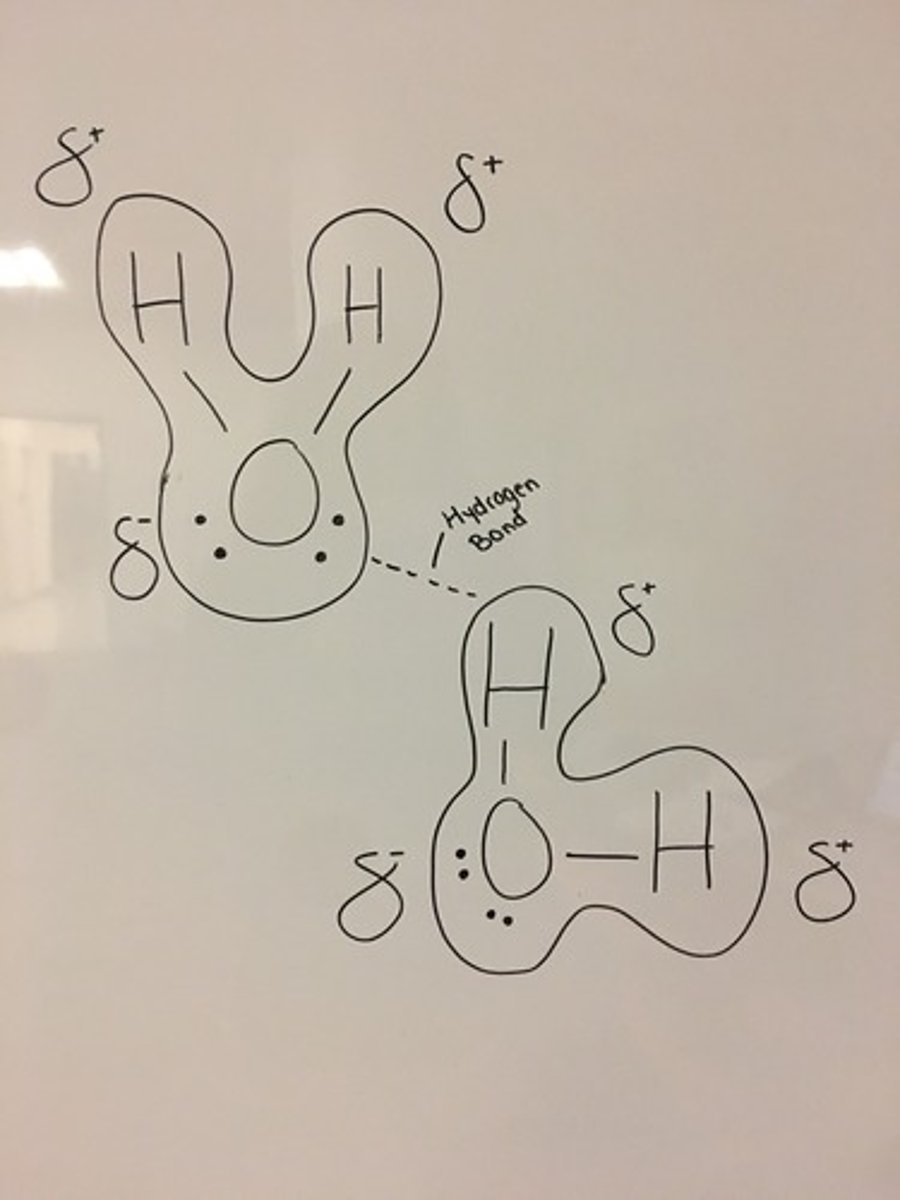
Is there a large electronegativity difference between H and N, O, F?
Yes
Which is strongest : London Forces, Permanent dipole-dipole forces, or Hydrogen bonds?
Hydrogen bonds
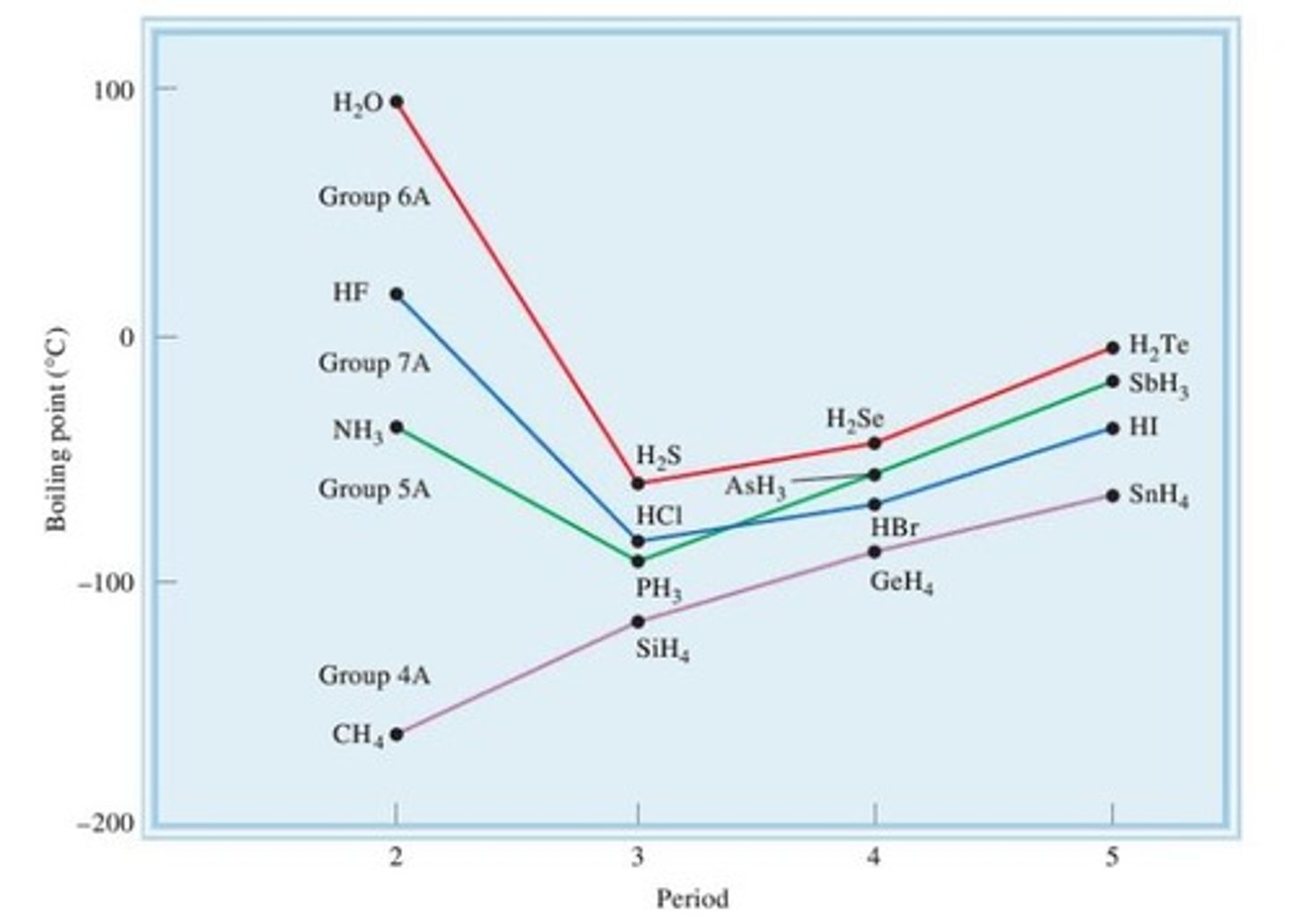
Explain this graph. Why does H₂O, NH₃, and HF, have anomalously high boiling points? Why is there a general increase from H₂S to H₂Te?
- The high boiling points of H₂O, NH₃ and HF are caused by the hydrogen bonding between the molecules.
- The general increase in boiling point from H₂S to H₂Te is caused by the increasing number of electrons, which in turn strengthening London Forces between molecules
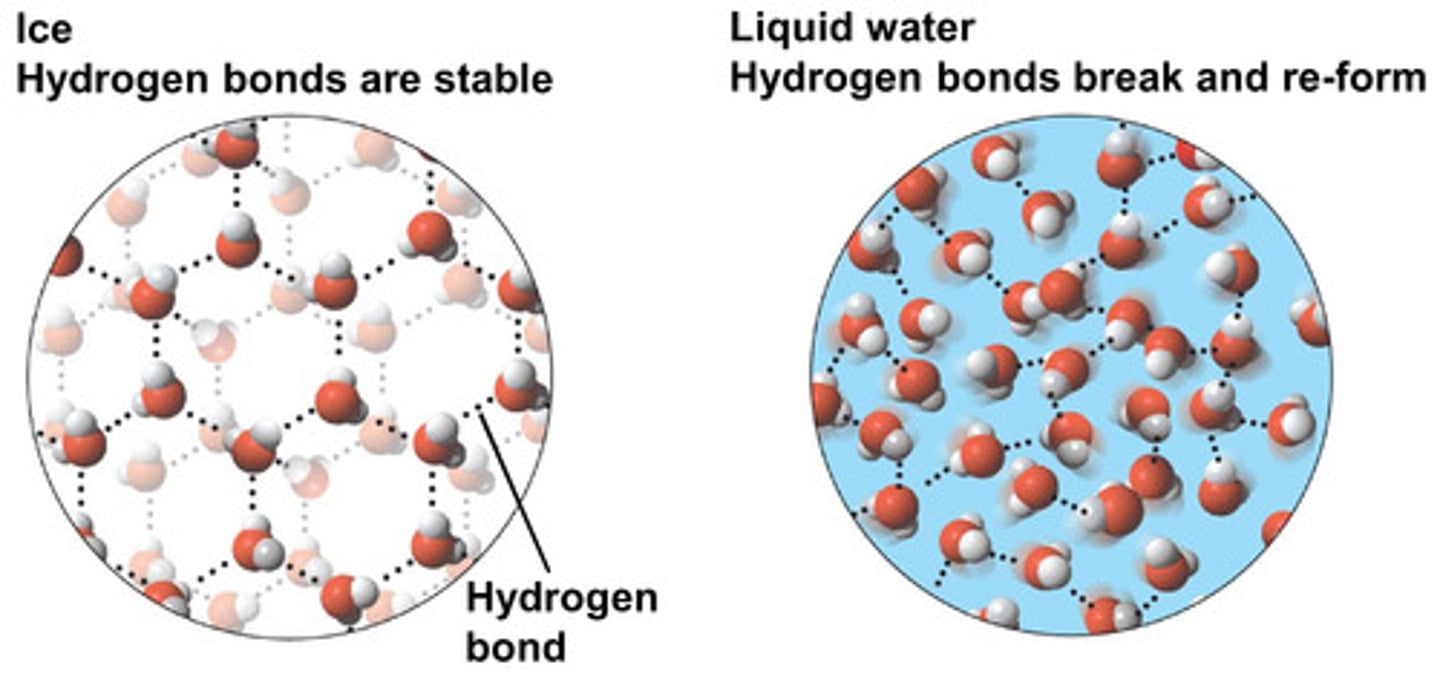
Why does ice have a lower density than water?
Water can form 2 hydrogen bonds per molecule because oxygen has 2 lone pairs.
This means that molecules are held further apart than in liquid.
Iodine
There are covalent bonds between the iodine atoms in the I₂ molecule.
Iodine crystals
The crystals contain a regular arrangement of I₂ molecules held together by weak induced dipole-dipole interactions (London forces) intermolecular forces