Lecture 3: Agonists to Inverse Agonists and Beyond
1/39
Earn XP
Description and Tags
Block 1
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
Nomenclature for Drug Targets
Agonists and Antagonists target receptors
~50% of drug targets are receptors
Activators and Inhibitors target enzymes
Opens and blockers target ion channels
Importance of Understanding Drug-Receptor Interactions
Allows for the development of drugs that can have positive/ inhibitory effects on the system of interest
This behaviour/effect is assessed in a test system (in vitro) e.g. by looking at the 2nd messenger or the response generated
Tests are carried out in-vitro using cultured cells or isolated tissues and receptors and can be used to predict the effects of drugs in a therapeutic system - they are cheap to carry out
can conduct in vivo, but these are complex systems and the results can be difficult to understand and they are expensive and regulated under legislation
Receptors
Proteins that detect specific molecules e.g. hormone, neurotransmitters
The endogenous agonist ligand activates this
Original View on How Drugs Interact with Receptors
Drugs either:
Agonists bind to a receptor and change its activity to produce an effect.
Antagonists: bind to a receptor but do not activate it, instead block the effect of agonists.
Affinity
Capacity of a ligand to bind to the receptor
Intrinsic Efficacy
Capacity of a ligand to excite the receptor and change its behaviour to produce a response
NB at the tissue/organism level, the ability of the drug to produce a response will depend on the number of receptors present as well as the intrinsic efficacy of the drug at the receptor
Rate of Dissociation
How quickly the drug comes off/dissociates from the receptor
Orthosteric Binding
Molecules that bind to the same site as the endogenous activator
Alloesteric Binding
Molecules that bind to alternative binding sites
PadLock and Key Analogy for Drug-Receptor Interactions
Padlock: Receptor
Key: Ligand
One padlock with 2 keys that both fit into the lock the effects following this differ
First key (Agonist): Fits the lock (receptor), is complementary, has affinity, and can open the lock (activates receptor). Has efficacy.
Second key (Antagonist): Fits the lock (receptor), has affinity, but does not open the lock (no activation). No efficacy.
Agonists
Have affinity and efficacy
They produce a measurable response following changes in receptor behaviour
2 Types
Antagonists
Have affinity but no efficacy
They do not product a measurable response wehen added alone
Partial Agonists
bind to receptors and produce a measurable response but do not produce a maximal response even when all receptors are bound (saturation).
The maximal effect is less than 100%.
There is a dose-dependent increase in response, but even at high concentrations, they do not achieve full receptor activation.
They:
Activate receptors.
Have affinity (bind to the receptor).
Have lower intrinsic efficacy than full agonists.
Do not produce the same maximal response as full agonists, even when receptors are fully saturated.
Act at the orthosteric binding site.
Constitutive Activity of Receptor
Receptors that display measurable activity even in the absence of a ligand.
This means the receptors can still carry out signal transduction and produce an effect, causing a low level of measurable activity or changes in cell behaviour without any external ligand binding
binding of full or partial agonists increases the receptors activation further leading to a bigger response
Inverse Agonists
Ligands that reduce/ suppress the constitutive activity of receptors.
They produce a response that is less than the baseline (i.e., less than the response seen without any ligand).
Show negative efficacy—only observable if the receptor has constitutive (baseline) activity.
Cause a dose-dependent decrease in basal activity; on a dose-response curve, effect starts high and ends low.
They:
Have affinity for the receptor.
Have intrinsic efficacy (but in the opposite direction to agonists).
Act at the orthosteric site (same site as endogenous ligands).
Full Agonists
Produce a 100% maximal response and lead to a dose-dependent increase in the measured response until the maximum is achieved.
They:
Activate receptors fully.
Have affinity (bind to the receptor).
Have intrinsic efficacy (capable of producing a response).
Are capable of producing a maximal response.
Bind to the orthosteric binding site, where endogenous agonists also bind.
Relationship Between Drug Binding and Receptor Behaviour
K+1 & K-1 are rate constants for binding and dissociation, determined by affinity.
α & β are rate constants for activation/inactivation, determined by intrinsic efficacy.
Receptors exist in two conformations: inactive (R) and active (R)*.
Agonists can stabilise the receptor in the active conformation (R)*, producing a response.
Drug A (Agonist):
Binds to the inactive receptor (AR) due to affinity, stabilises the active conformation, and produces a response.
coupled to the rate constants α and β that determine how stable and how long lived that particular active form is
Drug B (Antagonist):
Binds to the receptor due to affinity and forms a drug-receptor complex but does not stabilise the active form.
No response as it has no efficacy
Two-State Model of Activation
Receptors can switch between two conformations: resting (R) and active (R)* states.
The receptor is inactive in the resting state (R) and can produce a response in the active state (R)*.
The receptor's resting and active states are in a dynamic equilibrium.
In the absence of a ligand, the equilibrium lies heavily toward the resting state (R), meaning few receptors are active, and biological effects are negligible and can’t be measured
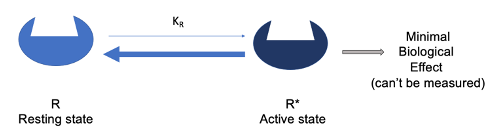
Difference Between Constitutively Active Receptors and Regular Receptors in the Two-State Model
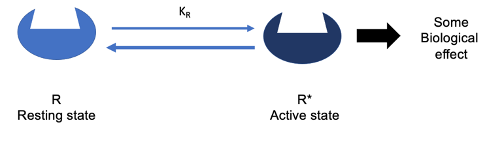
Constitutively active receptors have an equilibrium that lies further to the right, meaning they show a measurable level of activity even in the absence of a ligand.
While the receptor still exists in a dynamic equilibrium between resting and active states, the equilibrium for constitutively active receptors is slightly skewed toward the active state (R)*.
more receptors are in this active state
Despite this shift, most receptors are still in the inactive (resting) state (R), but enough receptors are in the active state to produce a measurable biological effect.
Two-State Model with Agonist Activiation
Equilibrium exists between the inactive (R) and active (R)* states of the receptor.
The number of receptors in the active state (R)* determines the biological effect/response.
Agonist (L) has a much higher affinity for the active state (R)* and a limited affinity for the inactive state (R).
Ligand binding stabilises the active state (R)*, shifting the equilibrium rightward—more receptors become active, resulting in a greater biological effect.
High ligand concentrations can shift nearly all receptors to the active state (R)*, producing a strong response.
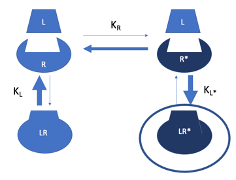
Two-State Model With Partial Agonist Activiation
Has a higher affinity for the active state (R)* but also some affinity for the inactive state (R) (though less than for the active state).
Binding can stabilize either state, but because the active state has a higher affinity, the equilibrium shifts toward the active state.
At high drug concentrations, most receptors are stabilized in the active state, but some receptors remain in the inactive state, meaning a maximal effect is never reached.
The net movement of equilibrium is to the right, increasing the proportion of active receptors and causing a dose-dependent increase in the response, but it never achieves a full response.
Buprenorphine
An analgesic that is used in the treatment of opioid addiction
Partial agonist at MOP (mu opioid receptors) – activated by morphine (opioid)
Acts as an antagonist to other opioids
Two-State Model Within Inverse Agonists
Has a higher affinity for the inactive state (R) than the active state (R*).
It stabilizes the inactive conformation, shifting the equilibrium to the left.
At high concentrations, most receptors are stabilized in the inactive state, leading to low or no biological response.
Inhibits constitutive activity (i.e., reduces baseline activity seen in absence of ligand).
Cimetidine
Histamine H2 receptor inverse agonist used to reduce gastric acid secretion
Histamine receptor are prone to constitutive activity
Pimavanserin
A highly selective serotonin 5-HT2A inverse agonist
attenuates psychosis in patients with Parkinson's disease with psychosis
Characteristics of An Endogenous Agonist
Typically a full agonist evolved to be highly efficient - optimised to produce a robust response
At maximal concentrations, it:
Fully activates the receptor.
Has high intrinsic efficacy.
Triggers full activation of all downstream signalling pathways.
Referred to as a balanced - activates all signalling pathways optimally
Super Agonists
A ligand that produces a maximal response that is higher than the receptor’s endogenous activator – rare
In response, the receptor to enter a distinct conformation that is highly efficient at producing a response (higher than 100%)
Isotonitazene
A synthetic opioid that has been linked to numerous deaths due to the recreational use - powerful opiate
Opiate receptors are prone to being activated by super agonist – activation above 100%, greater than endogenous agonists, morphine and even encephilkans
Biased Agonists
Ligands that activate only a subset of signalling pathways, not all equally.
They show functional selectivity: acting at the same receptor but preferentially activating certain responses over others.
Responses differ even though the same receptor is targeted.
They::
Have affinity and intrinsic efficacy.
Act at the orthosteric binding site.
May cause greater or lesser effects depending on the pathway they bias toward.
Oliceridine
A G-protein biased agonist at the µ-opioid receptor (MOP), a type of GPCR.
It preferentially activates G-protein signalling, leading to analgesia (pain relief).
It is less effective at activating β-arrestin pathways, which are associated with respiratory depression, nausea, and constipation.
Compared to balanced agonists like morphine it provides pain relief with fewer side effects.
Limitations of the Two-State Model
It is too simplistic - doesn’t suppourt the existence of super and biased agonist, which demonstrates that receptors are capable of existing in multiple different conformations
it remains as a useful tool for understanding drug-receptor interaction, but the reality is much more complex
Parameters Determined From Drug Binding Curves (Using Radioligand)
Measures how much radiolabeled drug binds to receptors in a tissue.
Helps determine:
Bmax: Maximum number of receptors present.
KD: Drug’s affinity for the receptor (concentration where 50% of receptors are bound).
KD is a key measure of affinity and is crucial in drug development for identifying potential drug candidates.
Parameters Determined From Dose-Response Curve
Measures the functional response to drug stimulation (e.g., contraction, secretion, Ca²⁺ release).
Cannot determine:
Affinity or
Number of receptors.
Can determine:
Emax: Maximum effect a drug can produce (efficacy).
EC50: Concentration of agonist that gives 50% of the maximal effect.
Helps identify if a drug is an agonist and if it can produce a measurable response.
Reasons for The Difference Between KD and EC50
There are multiple steps between receptor activation and the final measured response.
Some steps amplify the signal, while others may be inefficient.
As a result, KD (affinity) and EC50 (potency) are often not equal.
Often, only a small fraction of receptors need to be occupied to produce a maximum response—this is known as a receptor reserve or spare receptors.
Spare Receptors
They exist when Emax (maximum response) is achieved with less than 100% receptor occupancy (Bmax).
This occurs due to signal amplification—sometimes <5% receptor occupancy is enough.
If EC50 < KD, it suggests that 50% of the response is achieved with <50% receptor binding, indicating spare receptors.
The number of spare receptors varies by tissue, even for the same receptor, which can influence EC50 values.
Drug Potency and Its Relation to EC50
Potency refers to how much drug is needed to produce an effect.
EC50 is used to measure potency: lower EC50 = higher potency.
Potent drugs produce large effects at low concentrations.
Useful in drug development: lower dose = fewer off-target effects.
Potency depends on both affinity (binding strength) and efficacy (ability to produce a response).
For a partial agonist with an Emax of 80%, an EC50 of 40% would be expected.
Prenalterol
A β-adrenoceptor ligand with high affinity, low efficacy.
Acts as:
An antagonist in some tissues (e.g., guinea pig extensor digitorum longus) by blocking endogenous agonist effects.
A partial β-agonist in guinea pig left atria.
A full β-agonist in thyroxine-treated animals (e.g., guinea pig right atria) due to receptor upregulation, allowing enough activation for maximal response.
Its effect depends on receptor density and tissue sensitivity.
demonstrates how one drug can have different effecfts in different tissues
Alloesteric Modulators
Ligands that bind to the difference site on the receptor, the allosteric
It can bind at the same time as an orthosteric ligand
it will act to alter the affinity and or efficacy of the drug/ ligand binding at the orthosteric site
Positive (PAM) – enhance agonist effect
Negative (NAM) – suppress agonist effect
Silent (SAM) - no effect on agonist - prevents/interferes with the NAMS and PAMs from binding
Two State Model and Allosteric Modulators
Attempts to fit it to this model have been made but are complex and are nor representative of what actually occurs at the molecular level
Clinical Importance of Allosteric Modulation
Benzodiazepines (e.g. Diazepam) are classic allosteric modulators of GABA (endogenous agonist) at GABAA receptors
Potential of muscarinic acetylcholine PAMs in the treatment of Alzheimer’s disease
Potential of m-opioid receptor NAMs in opioid use disorder/ opiate abuse