KAPLAN MCAT ORGANIC CHEMISTRY CHAPTERS 1-12
1/193
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
194 Terms
Heteroatoms
atoms in a molecule that are not carbon and hydrogen
oxidation state changes with respect to heteroatoms
- increases with more bonds to heteroatoms
- decreases with more bonds hydrogen
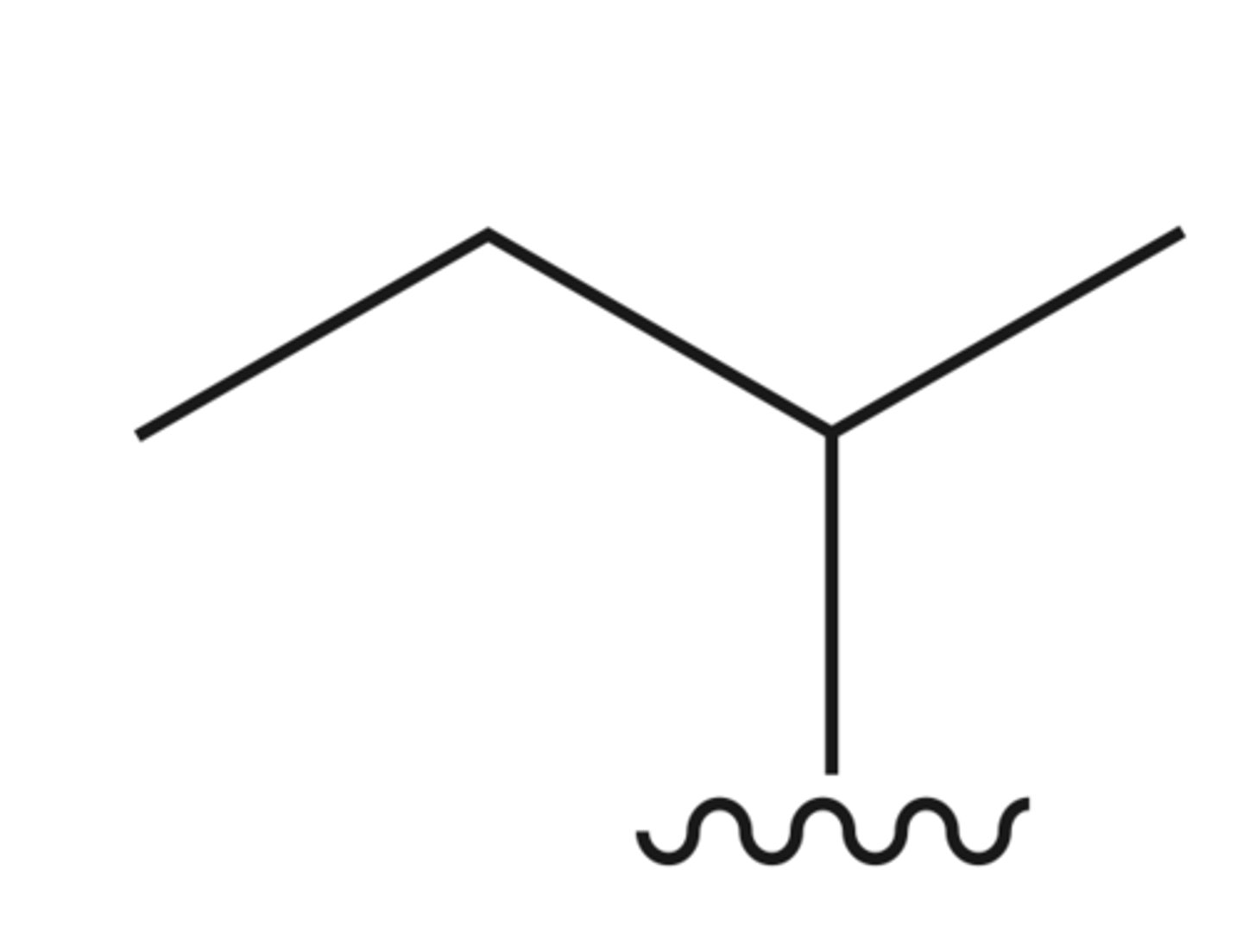
sec-butyl
neo-pentyl
Diols also called and what are they
-alcohols with 2 hydroxyl groups
aka glycols
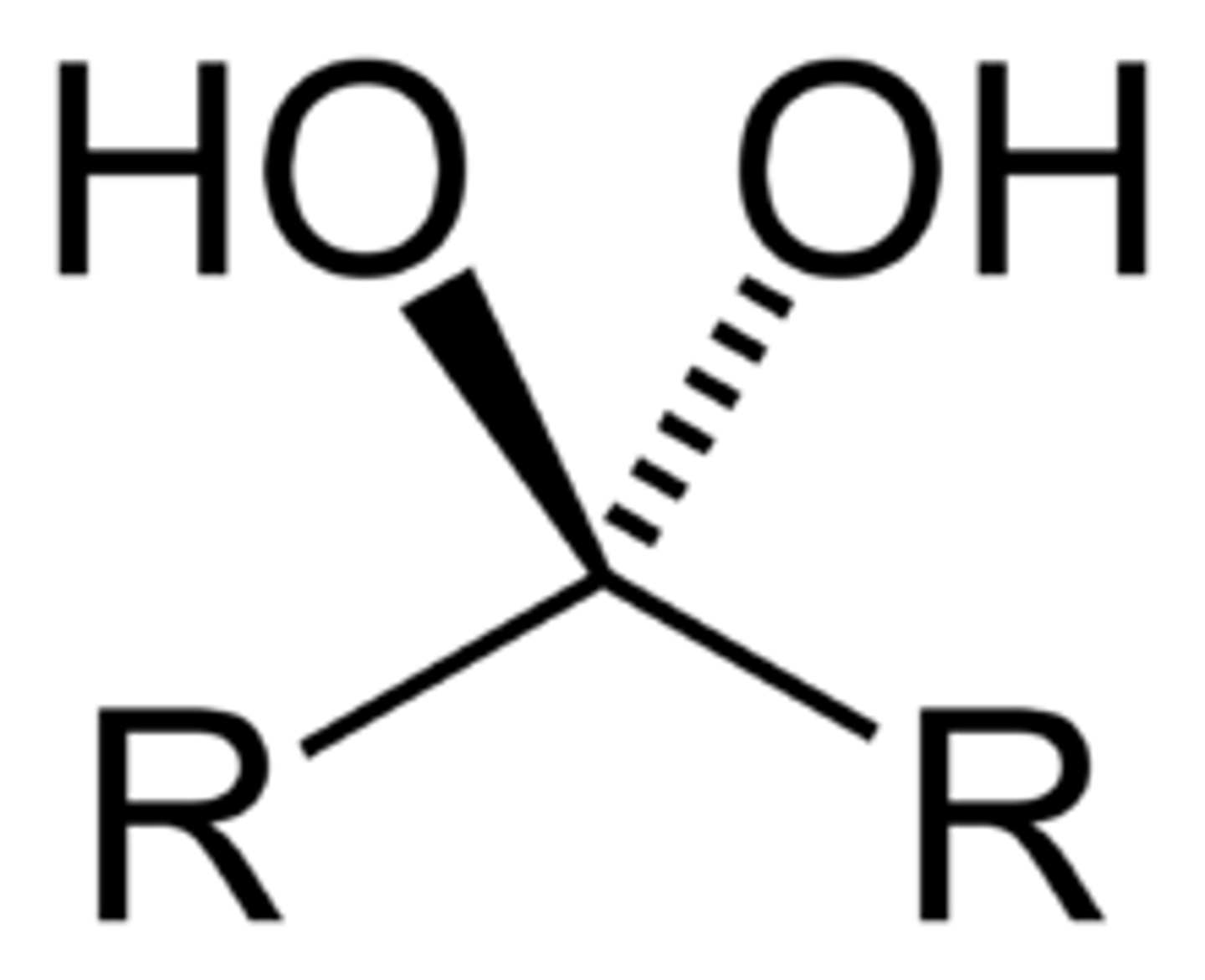
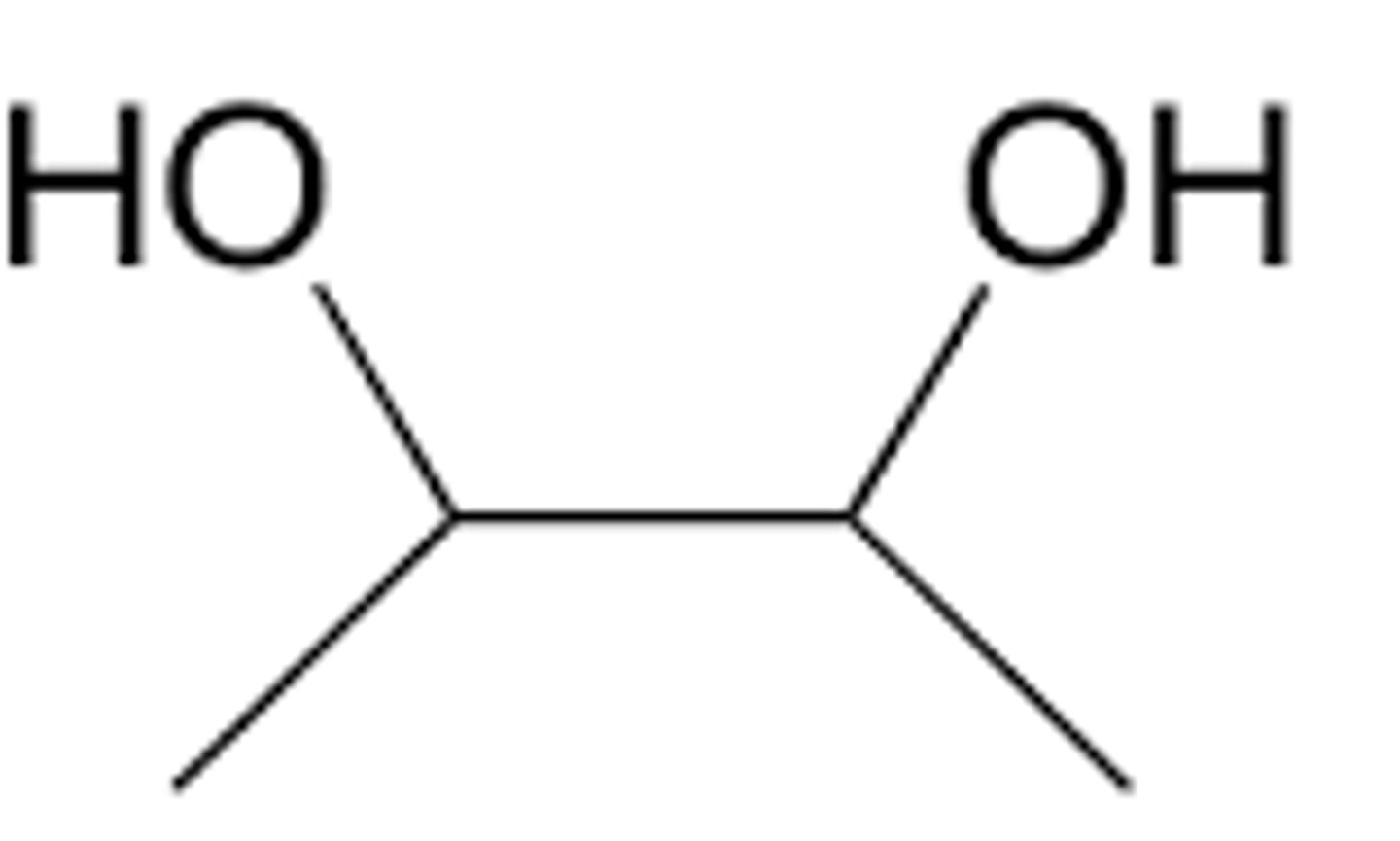
geminal diols
-diols with hydroxyl groups on the same carbon
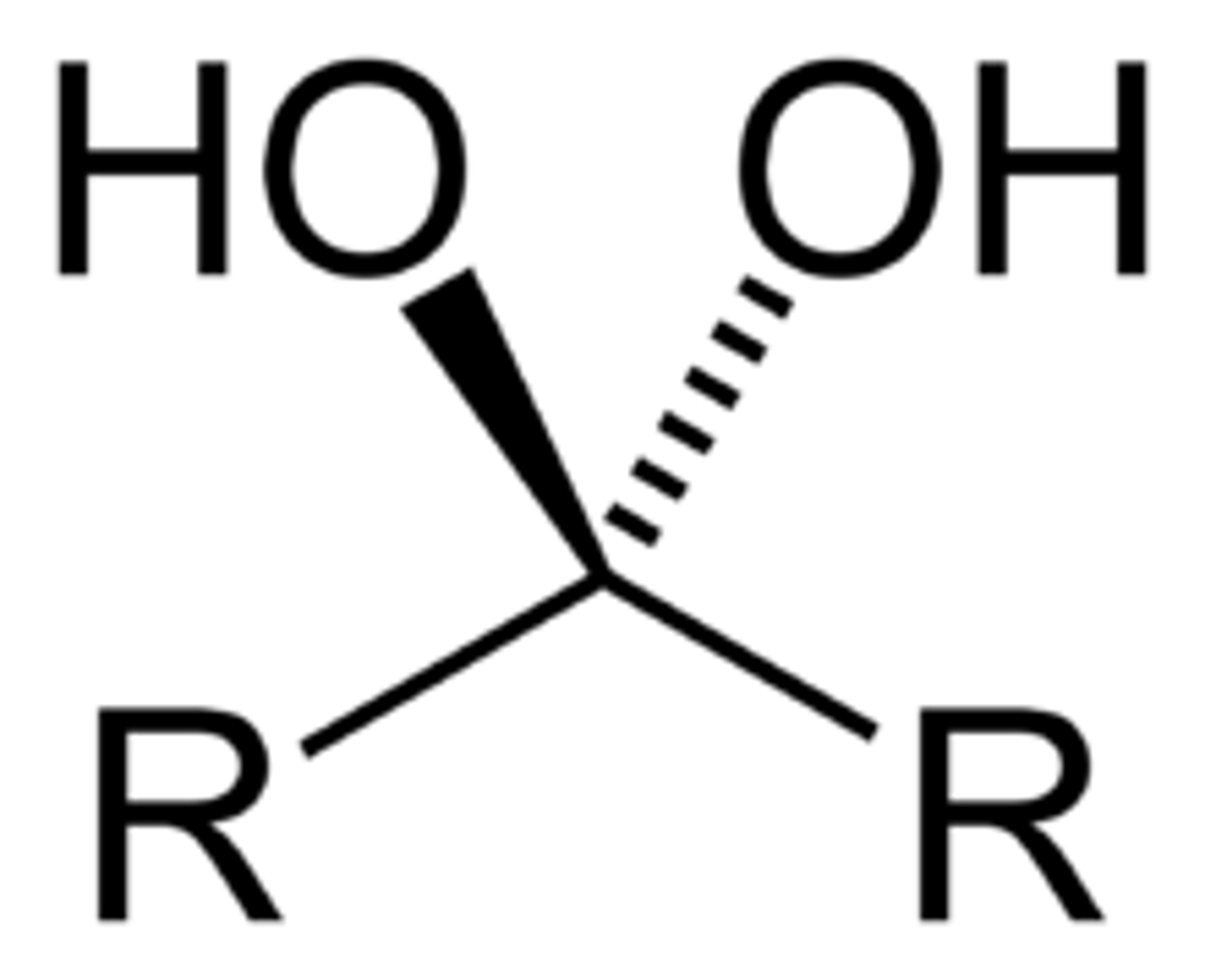
vicinal diols
-diols with hydroxyl groups on adjacent carbons
like NEXT to each other, in the same VICINity
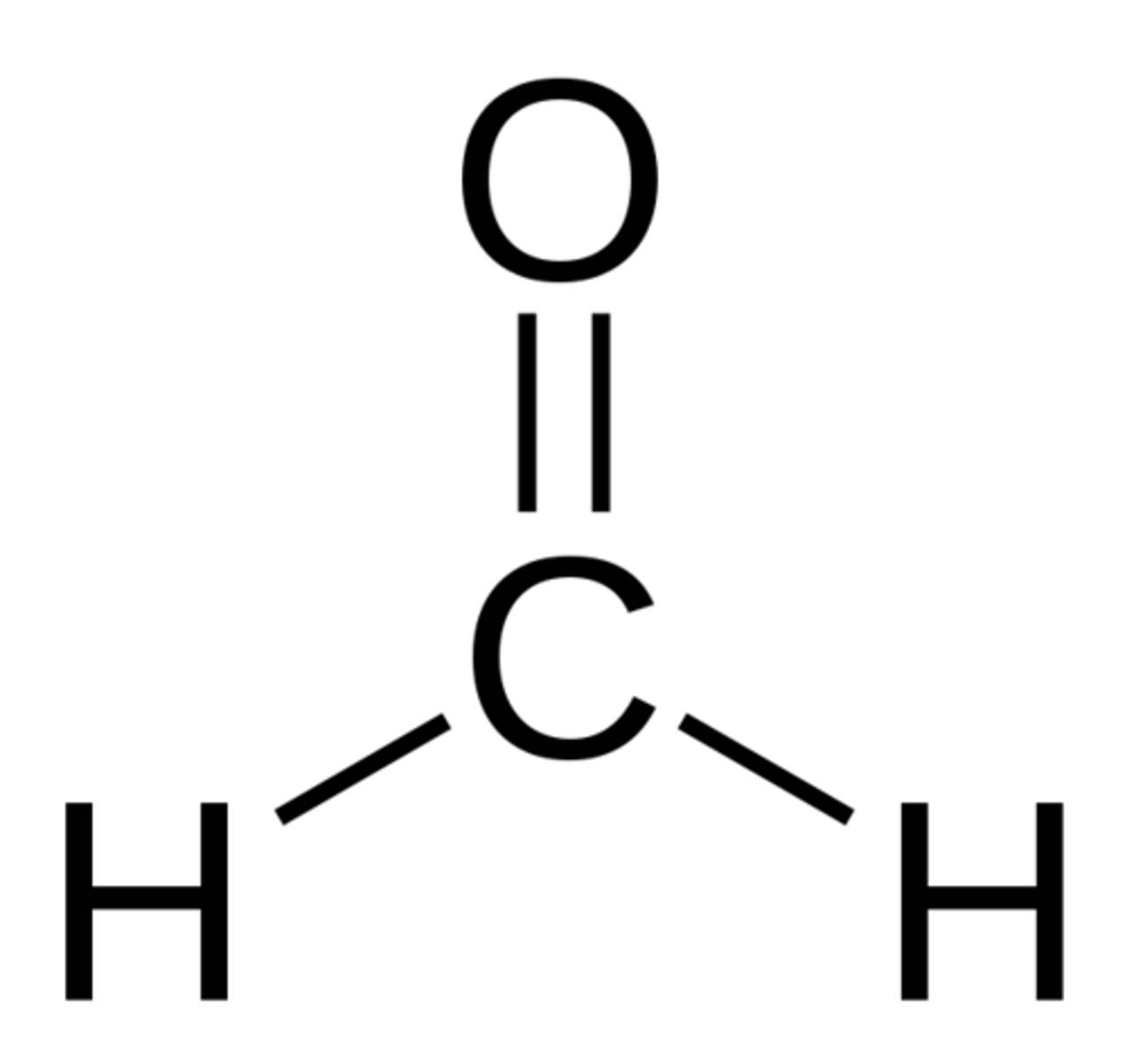
Formaldehyde
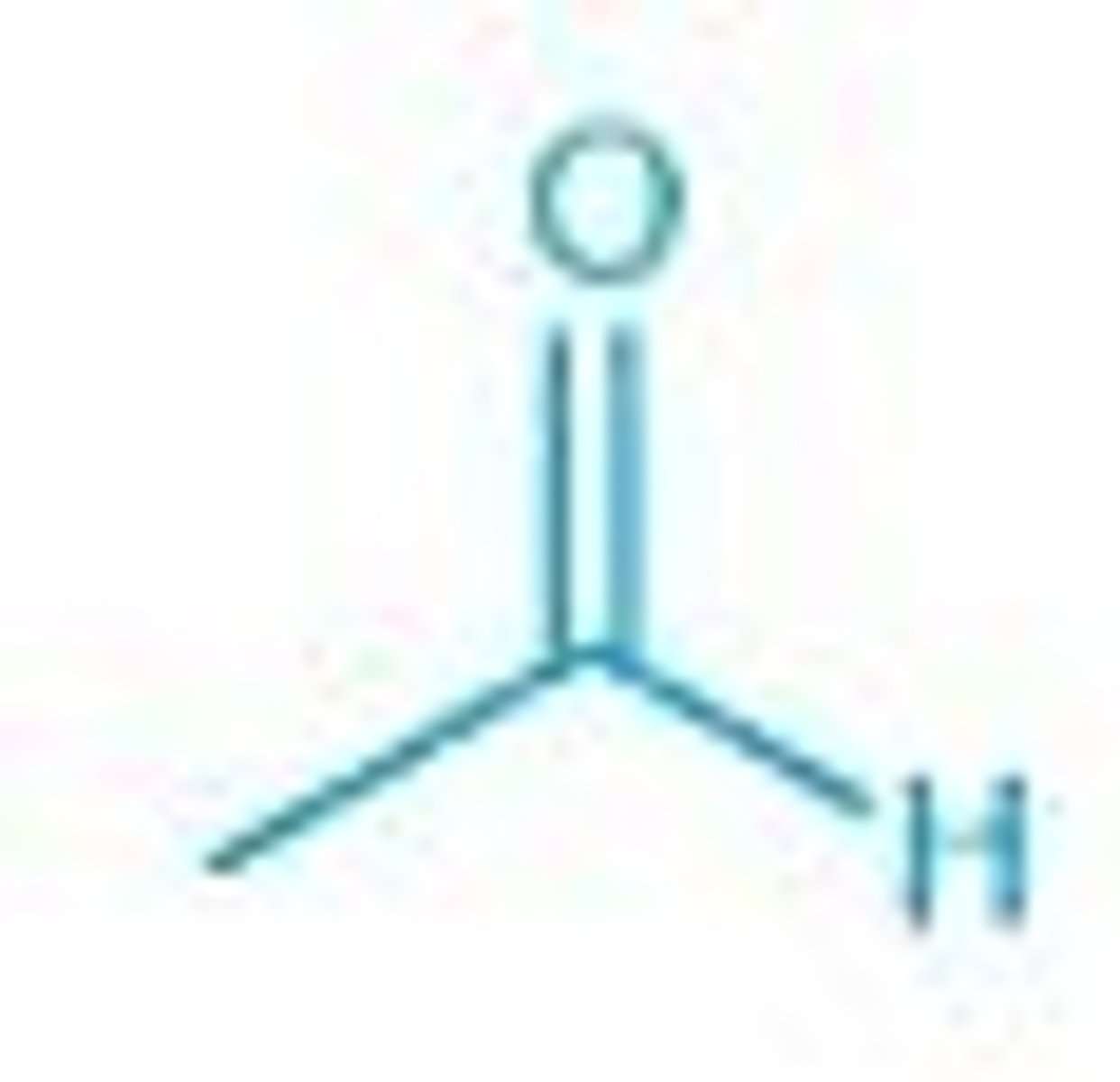
acetaldehyde
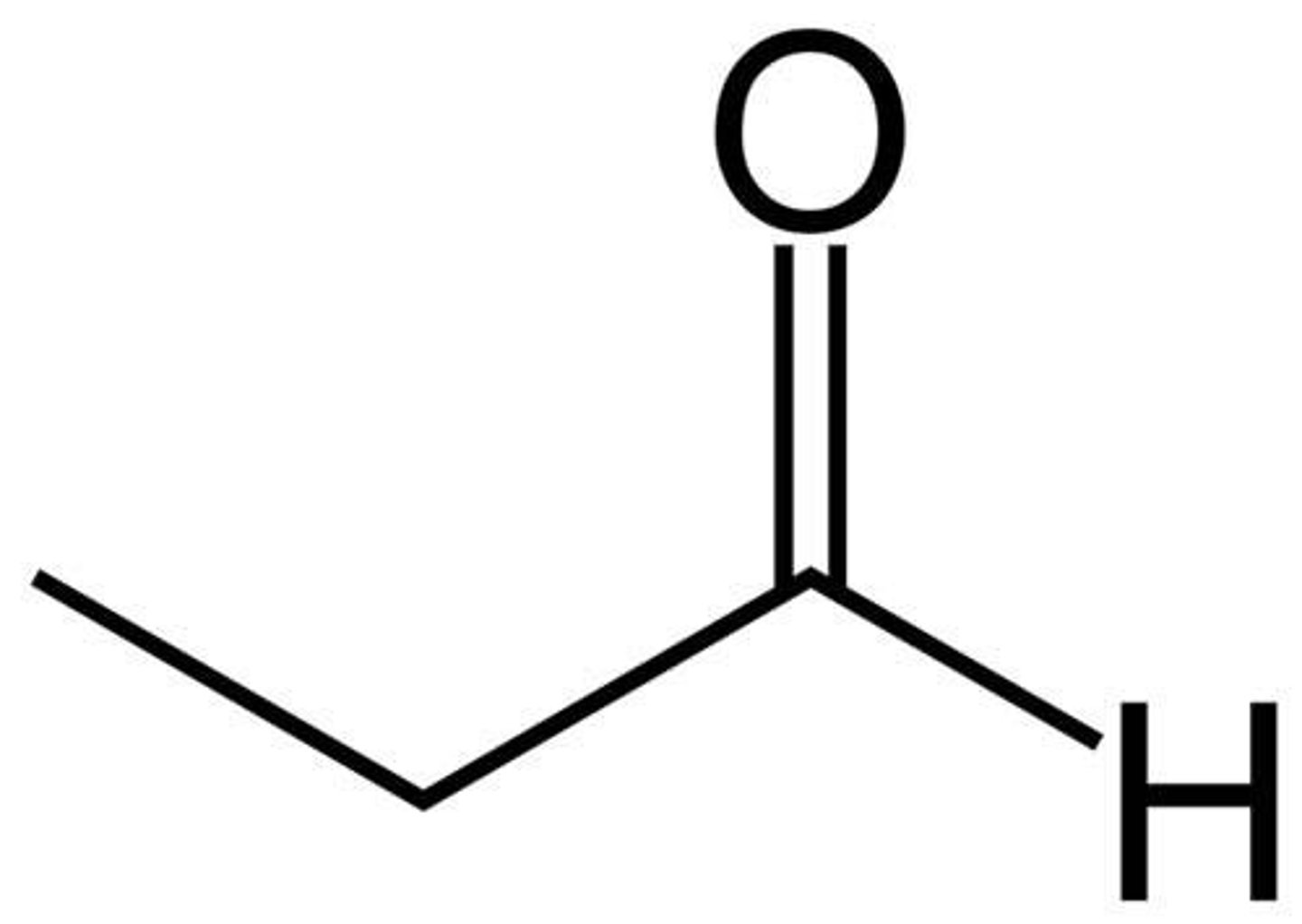
propionaldehyde
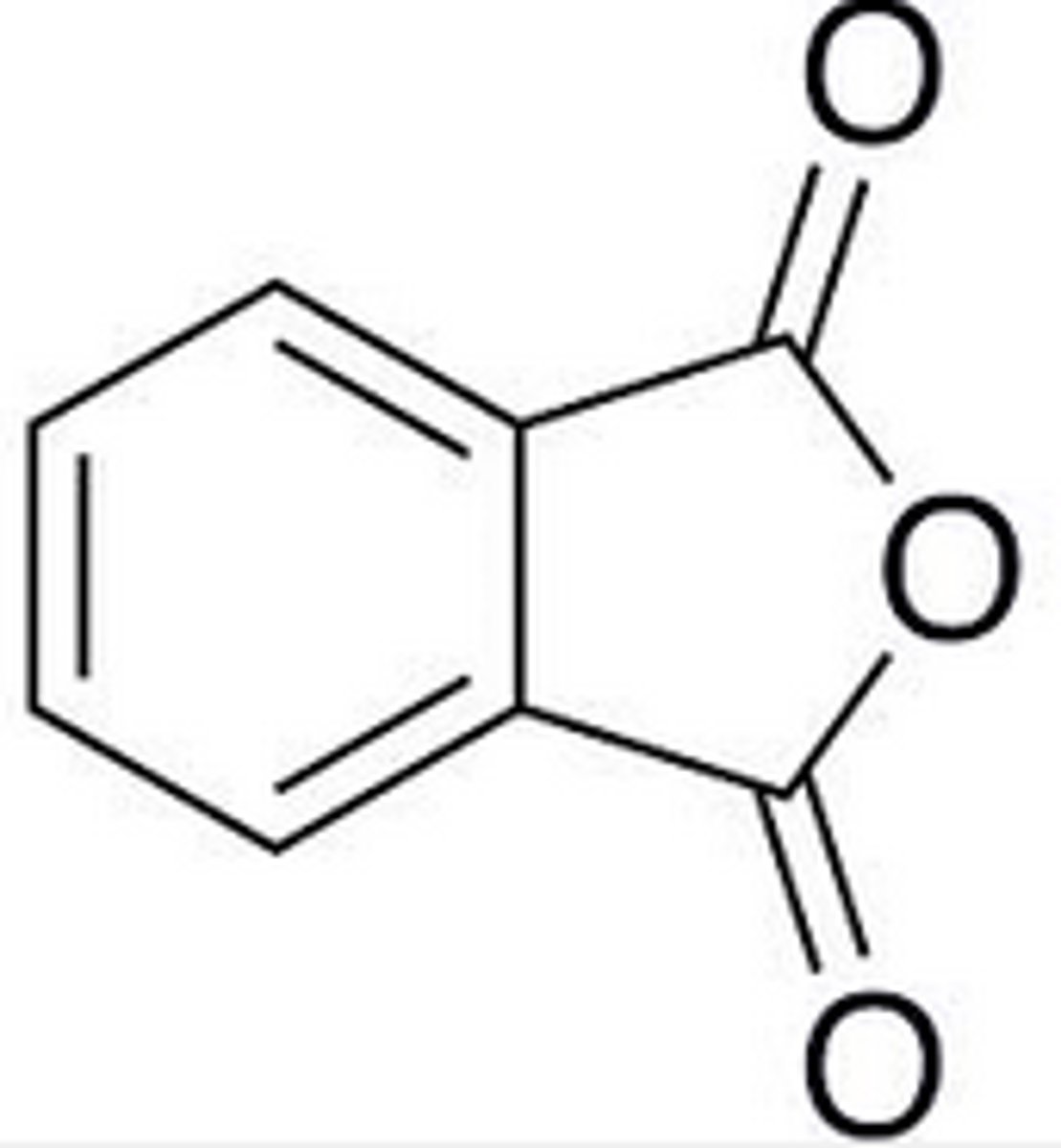
phthalic anhydride
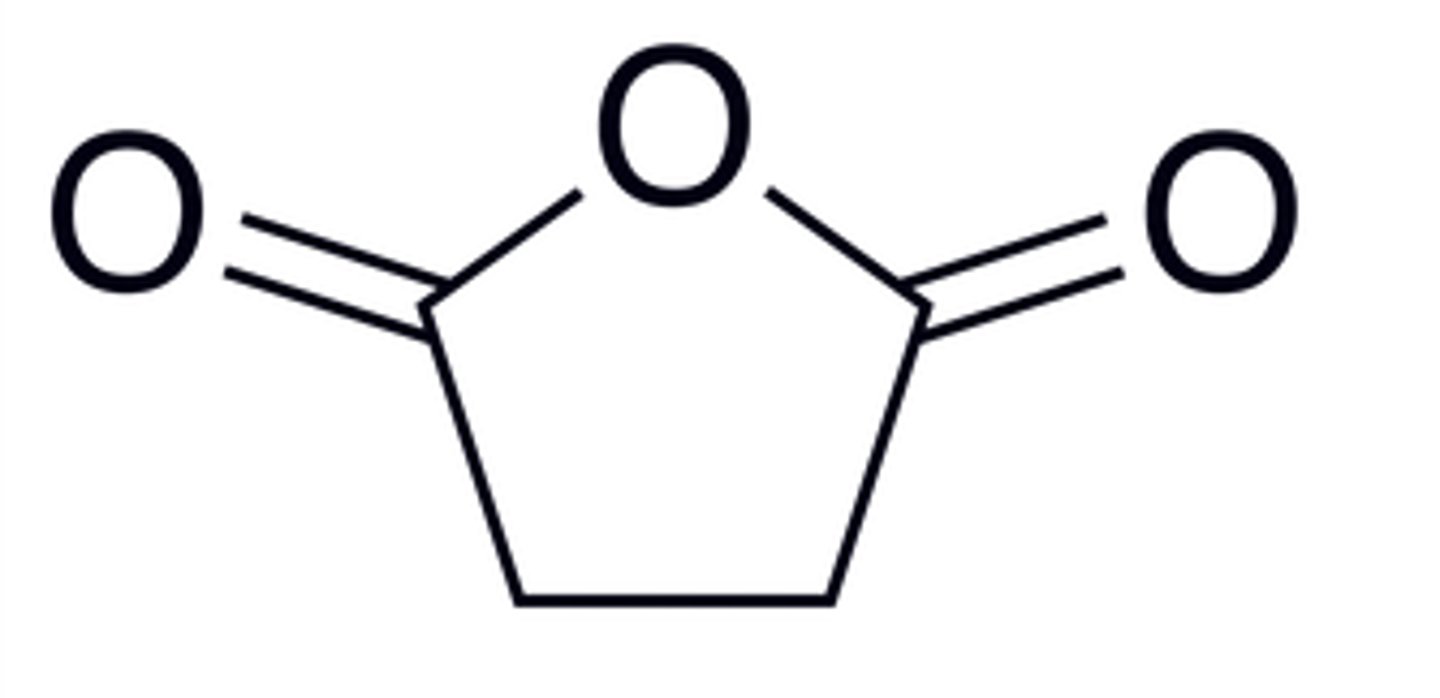
succinic anhydride
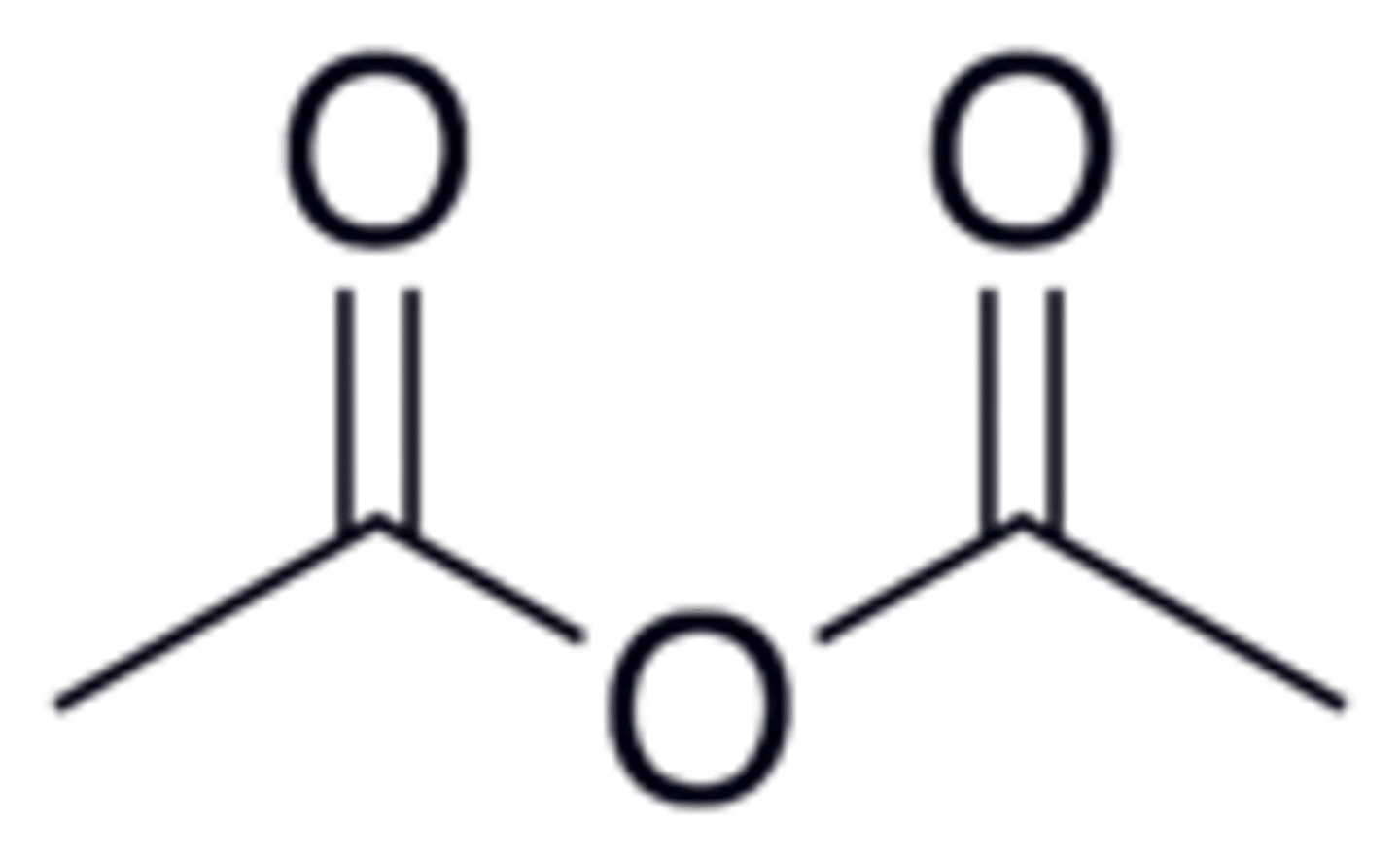
acetic anhydride
Major functional groups, give prefix and suffix for naming
*highest oxidation state to lowest:
1) carboxylic acid
2) anhydride
3) esters
4) amides
5) aldehydes
6) ketones
7) alcohol
8) alkenes
9) alkynes
10) alkanes
physical properties
- characteristics of a substance that can be observed or measured without changing the substance
-EX: mp, bp, odor, density, color, solubility
chemical properties
Characteristics of a substance that determine how it will react with other substances.
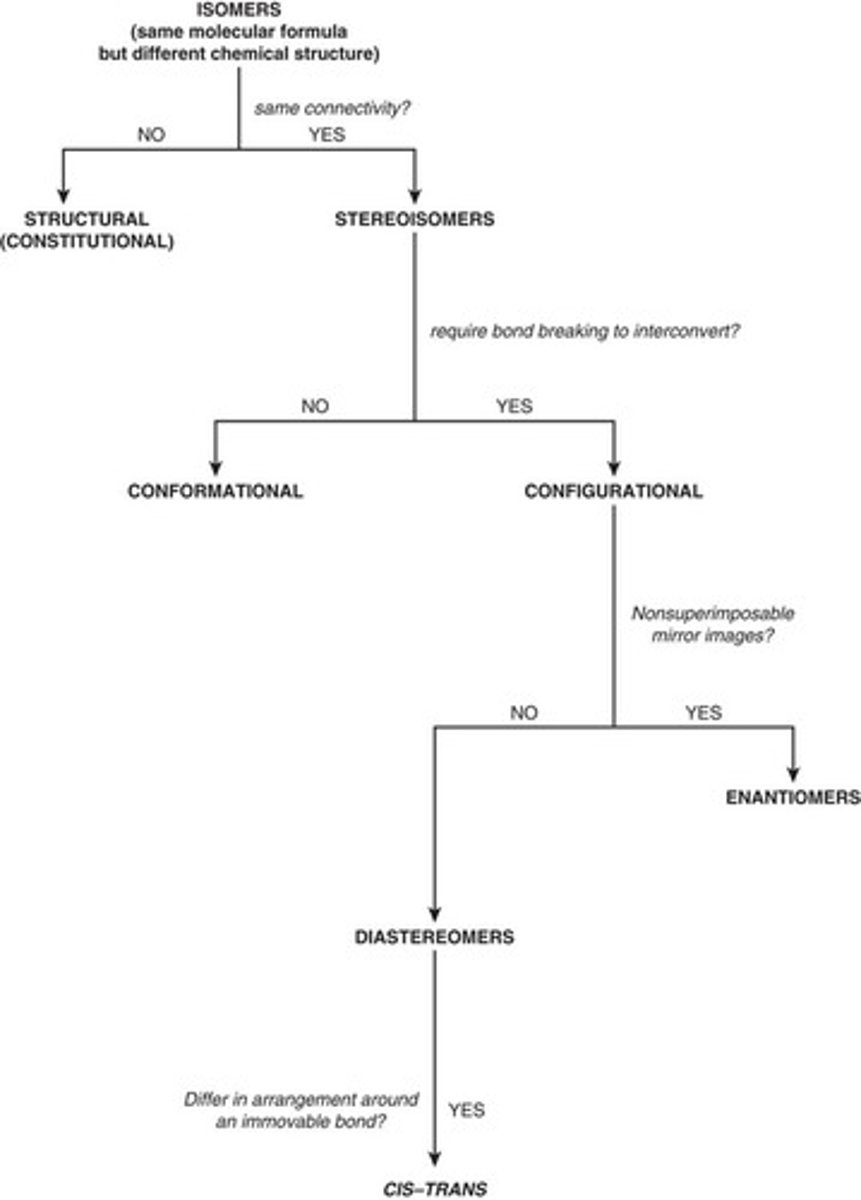
Isomers
Compounds with the same formula but different structures.
EX: C5H12 with two different structure
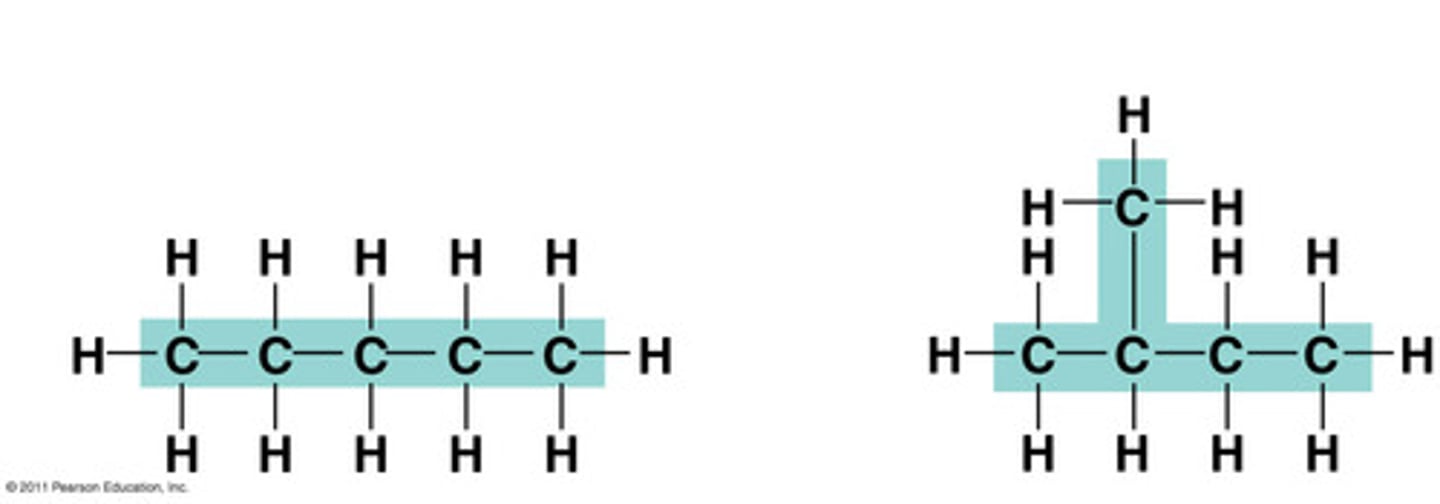
structural (constitutional) isomers
-same molecular formula, different connectivity
-same molecular weight
Flowchart of Isomer Relationships
Stereoisomers
-same chemical formula.
-same atomic connectivity
-different in how atoms are arranged in space
-slow at low temperature

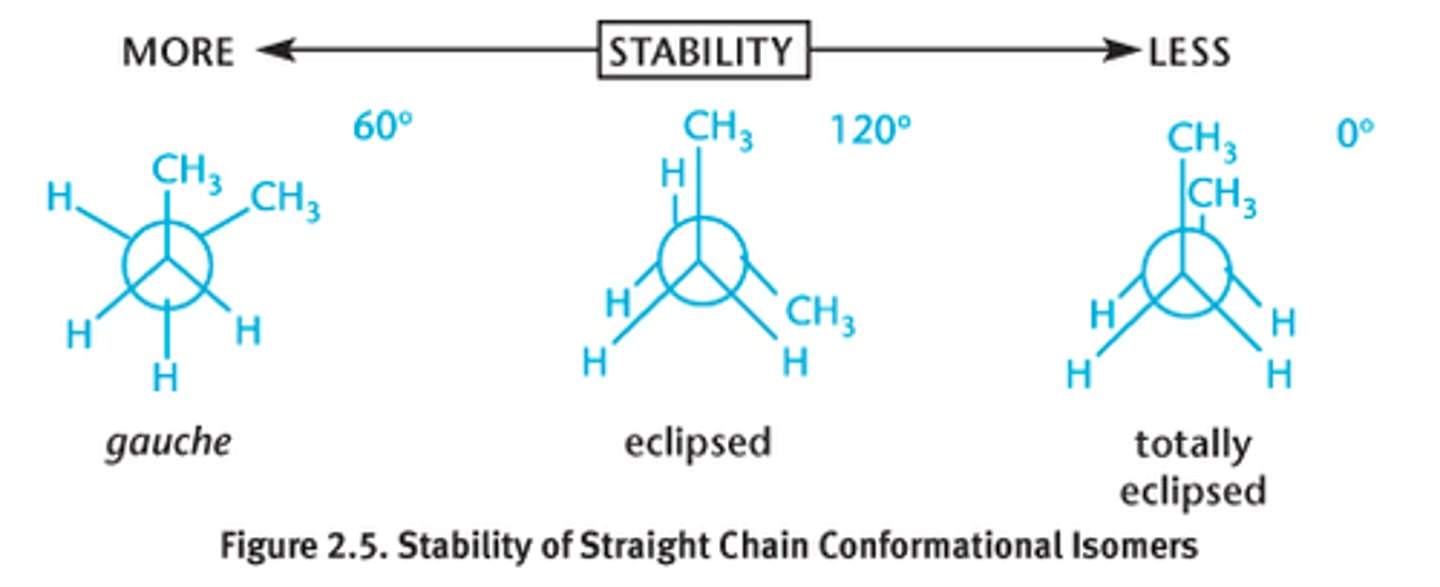
conformational isomers
-differ by rotation around a single sigma bond
-most similar
-same molecule
Cycloalkanes
-can be stable or unstable depending on ring strain
-Ring strain arises from 3 factors:
1) angle strain:
-results when bond angles deviate from their ideal values by being stretched or compressed
2) Torsional strain
-when cyclic molecules must assume conformations that have eclipsed or gauche
3) Nonbonded strain (van der waals repulsion):
-when non adjacent atoms compete for the same space
chair conformation
-The chair-shaped conformation of cyclohexane that has no angle strain and has no torsional strain because it is perfectly staggered about all the C-C bonds. It is strain free.
-bulkiest group favor equatorial position to reduce nonbonded strain (flagpole interactions) with axial groups
configurational isomers
- interconverted only by breaking bonds and reforming covalent bonds
what makes a molecule chiral
An object is considered chiral if its mirror image cannot be superimposed
on the original object
what is a chiral center
has four different substituents attached to the atom
what can chiral centers and chiral molecules not have
a plane of symmetry
What are Enantiomers
non super imposable mirror images
how are enantiomers identified
have the same connectivity but opposite configurations at every chiral center in the
molecule
what is true about the properties of a pair of enatiomers
Enantiomers have identical physical and chemical properties except: optical activity and reactions in chiral environments
what is optical activity
the ability to rotate plane polarized light
describe light rotation for enantiomers
rotates plane-polarized light to the same magnitude but in the opposite direction of its mirror image
(assuming concentration and path lengths are equal)
rightward/ clockwise location name and sign
d, and +
leftward rotation name and sign
l and -
formula for specific rotation
[a]= a obs (observed rotation)/ c (concentration) times l (path length)
Racemic Structure
-when (+) and (-) enantiomers are present in equal concentrations
--->>>rotations cancel each other out and NO OPTICAL ACTIVITY is observed
-reacting 2 enantiomers with single enantiomer of another compound, lead to 2 diastereomers
EX: (+) and (-) reacted with (+)enantiomer each---->(+,+) and (-,-) diastereomers
what are diasteroemers
non superimposable non mirror images
how to detect a diastereomer
a molecule has two or more stereogenic centers
and differs at some, but not all, of these centers
Meso compounds
-Optically inactive (achiral) molecule with two chiral centers
-have a plane of symmetry
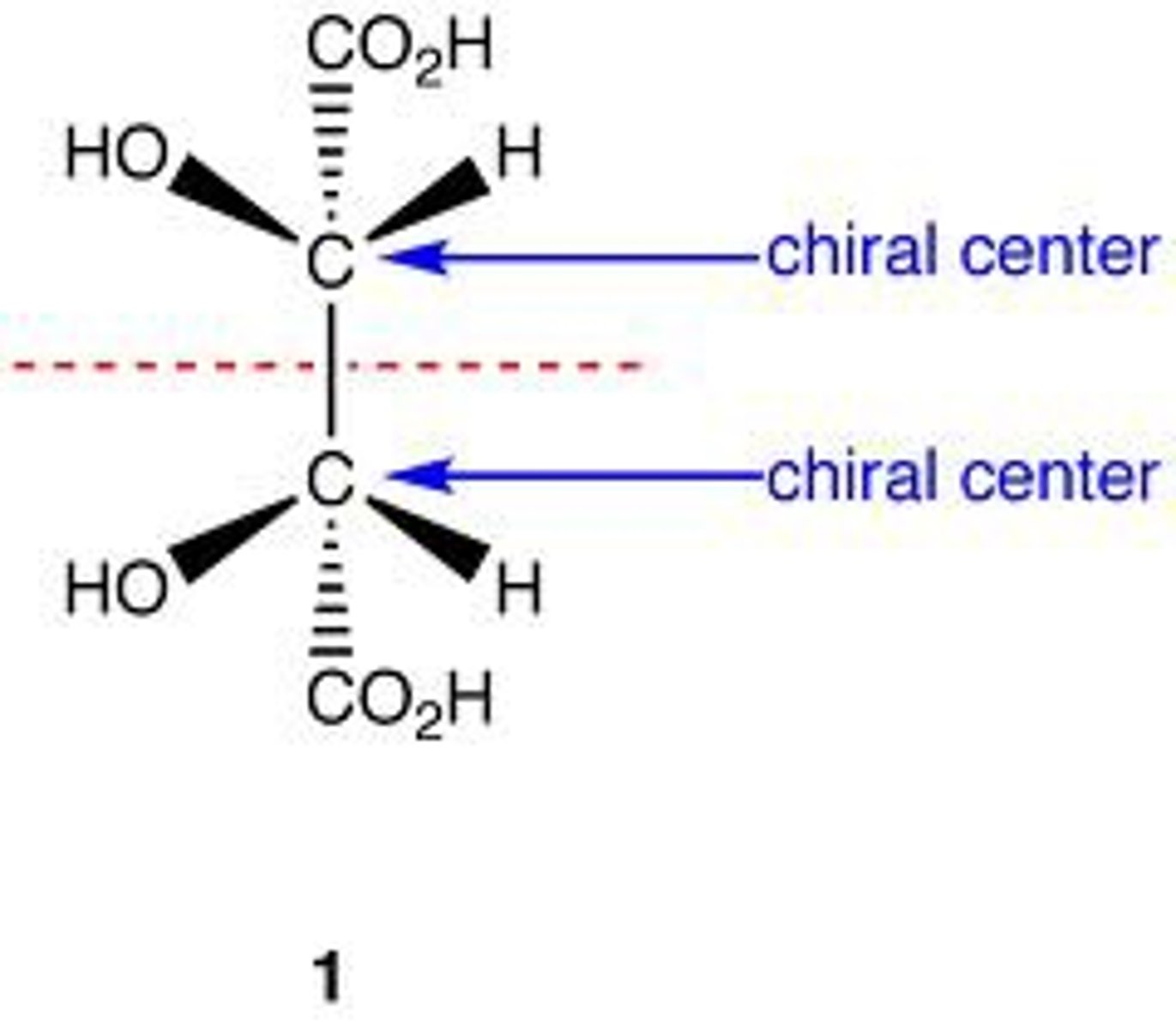
what is true about diastereomer and the plane of polarized light
knowledge of the specific rotation of one diastereomer gives no indication of the specific rotation of another diastereomer. completely different from enantiomers
subtype of diasteromers
what are the types
what is the meaning of each type
cis tran trans isomers aka geometric isomers
Hs on same side= cis
Hs on opposite sides of double bond= trans
what are characteristic of me so compounds
what does this casue
For a molecule to have optical activity, it needs to have chiral centers
within it, but must also lack a plane of symmetry.
if a plane of symmetry exists, the molecule is not optically active even if it possesses
chiral centers.
the plane of symmetry can happen either through the chiral center or between chiral centers.
A molecule with chiral centers with an internal plane of symmetry is called a meso compound,
how to determine E/Z isomers and what they are
there is a double bonded carbon
identify the highest-priority substituent attached to each double-bonded
carbon.
the higher the atomic number, the higher the priority.
If the atomic numbers are equal, priority is determined by the next atoms outward; again, whichever group
alkene is named (Z) “zee zame zide’— German accent— if the two highest-priority substituents on each carbon are on the same side of the double bond and
E if the highest priority substituents are on opposite sides of the double bond
How to determine R vs S configurations
what to when lowest priority atom is a wedge
what to do when lowest priority atom is in plane
fischer projection stuff chapter 2.3
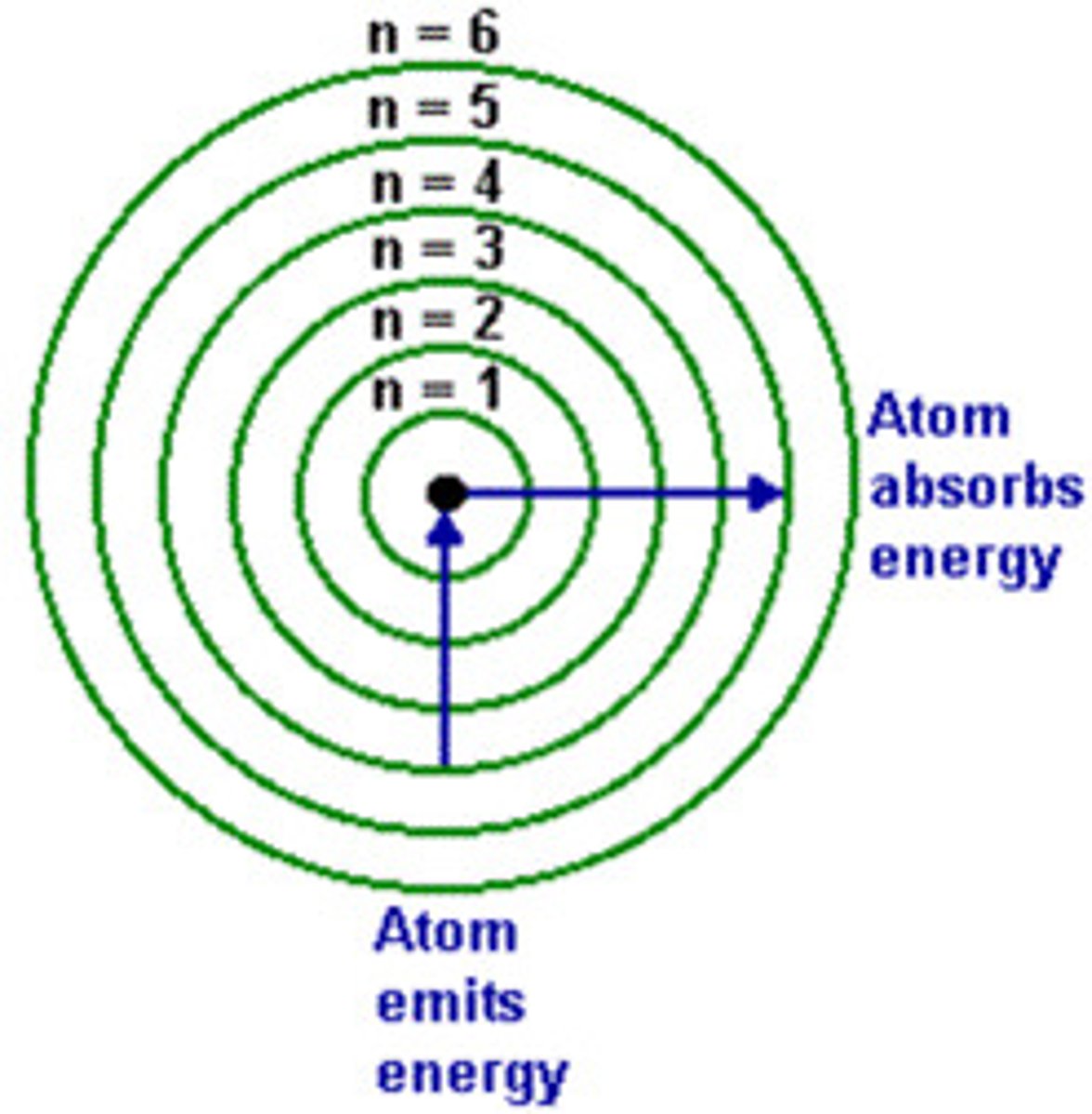
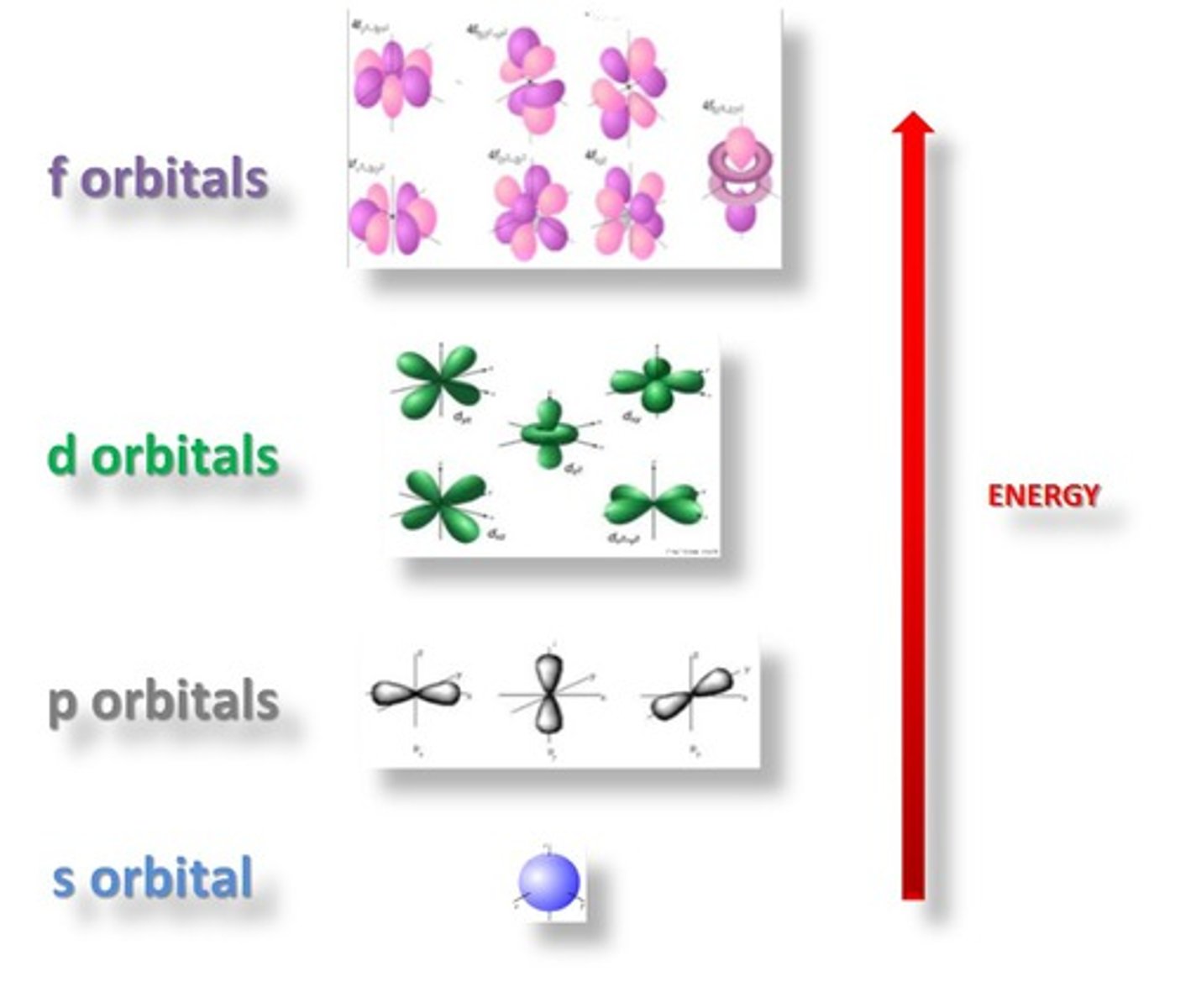
principal quantum number: n
- energy level of a given electron in an atom
- is a measure of size
- smaller number = closer the shell is to the nucleus and has lower energy
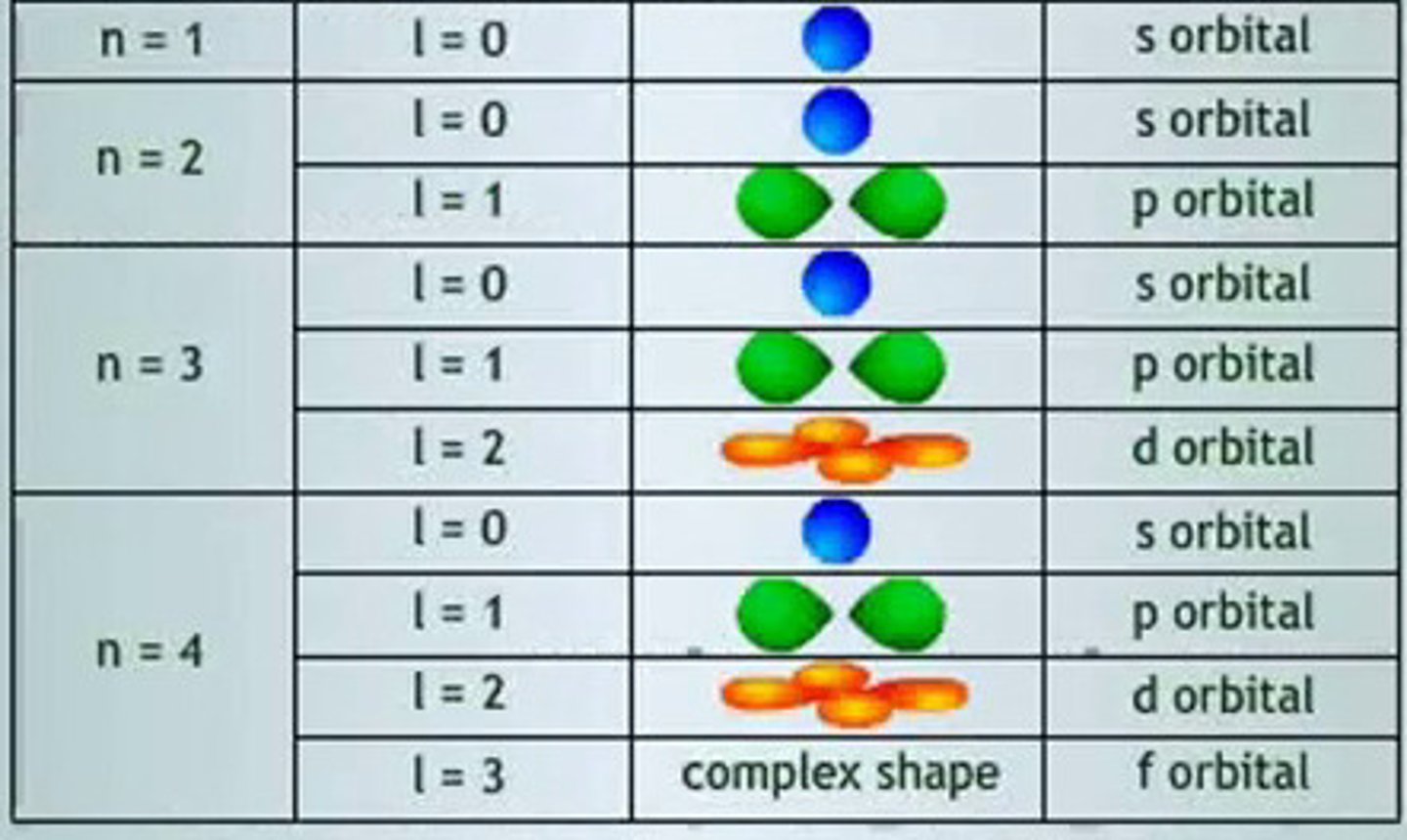
azimuthal quantum number: l
- ranges from 0 --> (n-1)
-energy increase as "l" increases
*describes subshells:
- 0: s subshell
- 1: p subshell
- 2: d subshell
- 3: f subshell
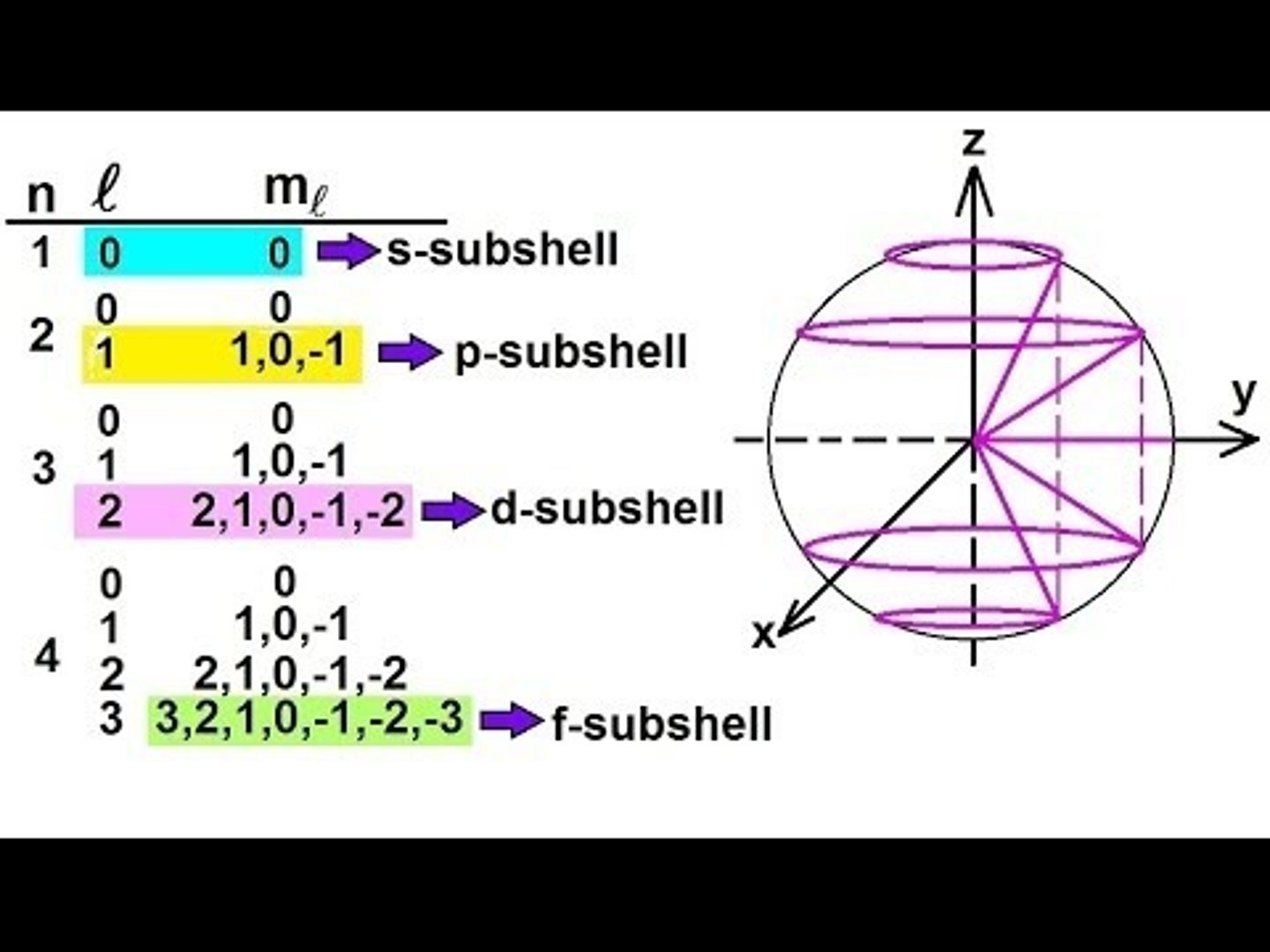
magnetic quantum number: ml
describes orbitals
- ranges from -l --> +l
spin quantum number: ms
distinguishes one of the two electrons in a particular orbital
- 1/2
- -1/2
Orbitals
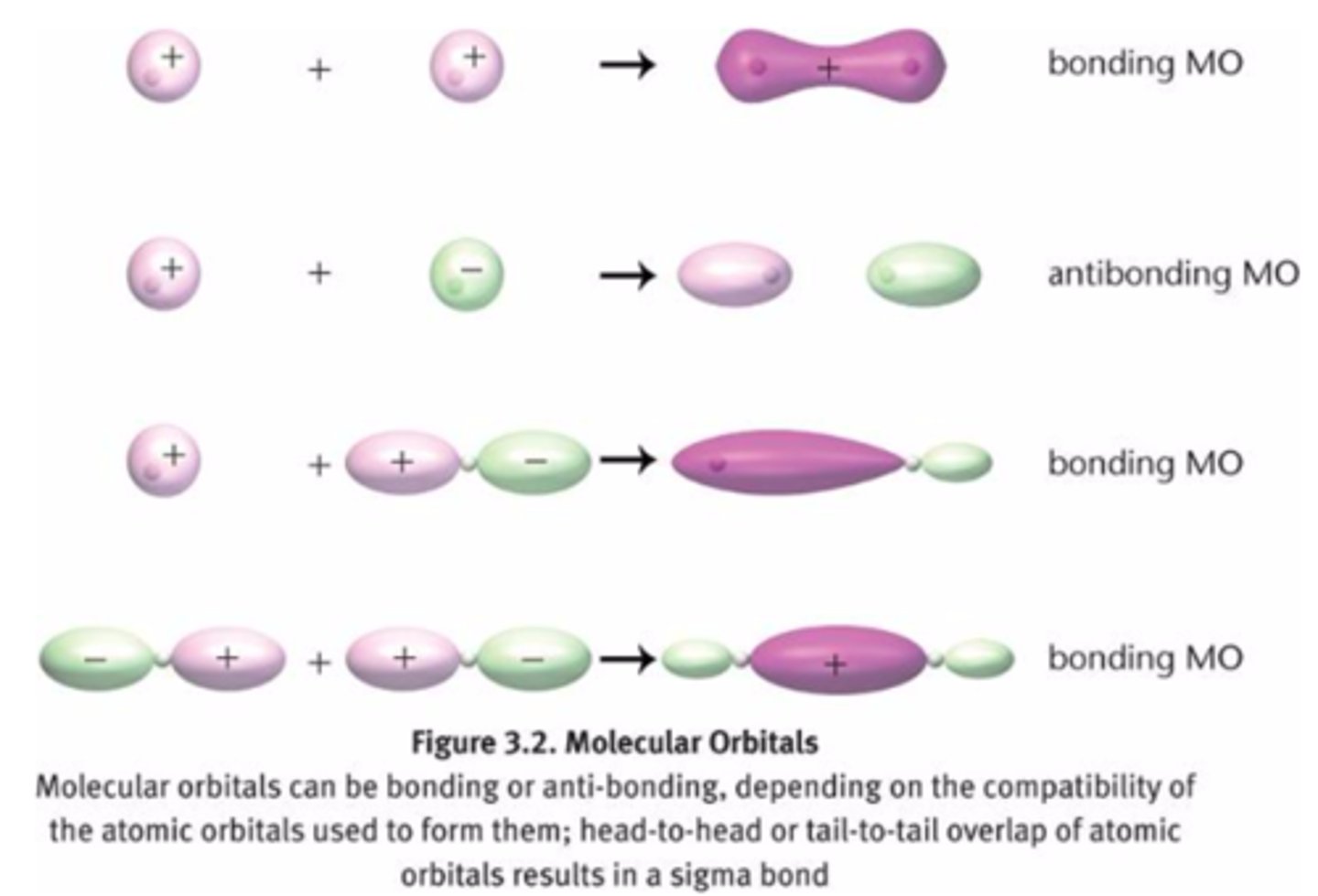
Molecular Orbitals formation, types and energy differences
-formed when two atomic orbitals combine
-Bonding orbitals: lower in energy (more stable), combining of waves of the same sign
-Antibonding orbitals: higher energy (less stable), combining of waves of opposite signs
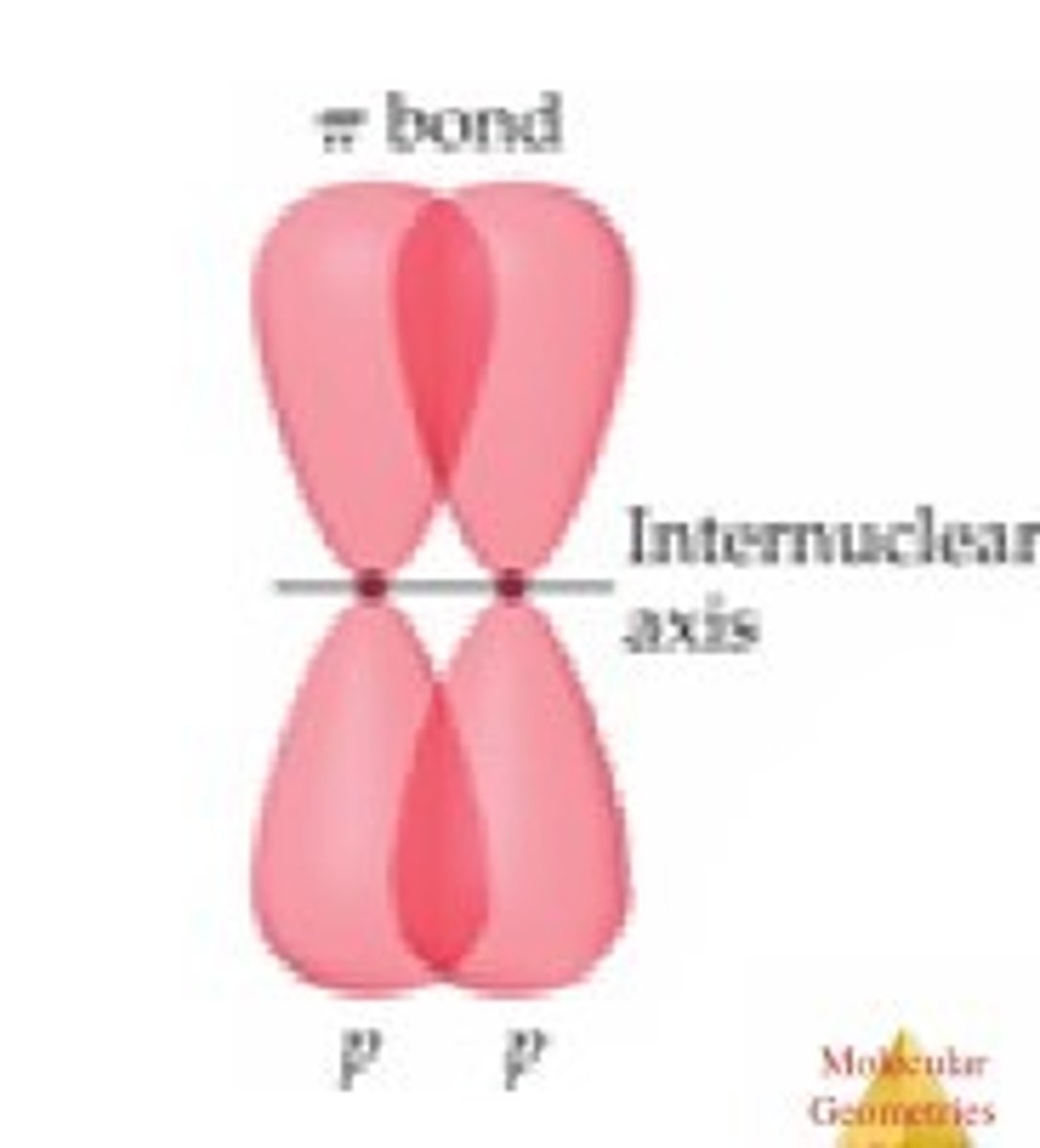
π bond and sigma bond and strength differences
-when two p-orbitals line up in a parallel fashion, electron clouds overlap + a π bond is formed
-weaker than sigma
sigma bond is formed from head to head or tail to tail overlap
all single bonds are sigma bonds
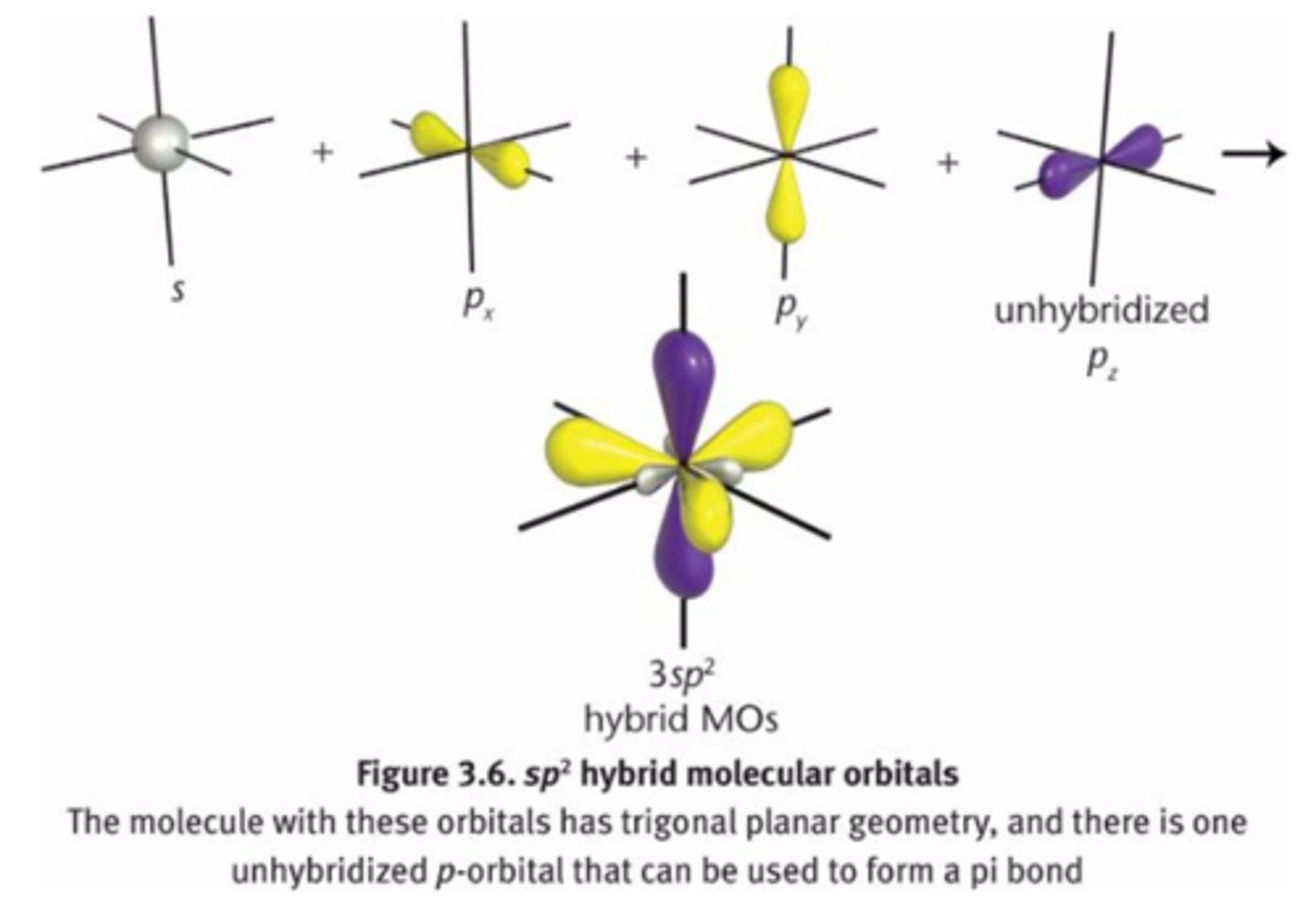
hybrid orbitals
formed by mixing different types of orbitals
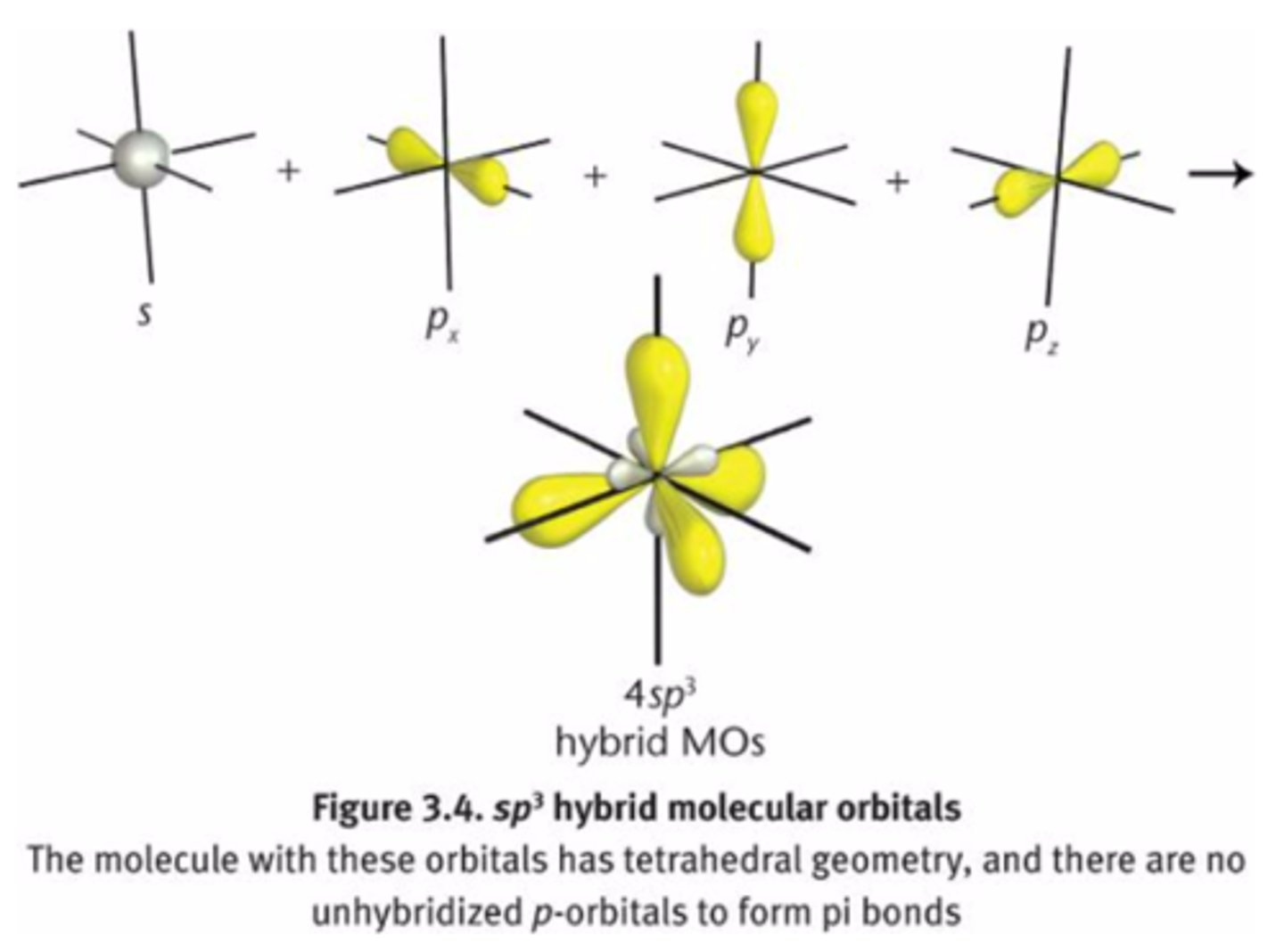
sp3 orbitals
-single bond
-tetrahedral
25% s character
75% p character
more p character = longer bond
more s character = shorter bond
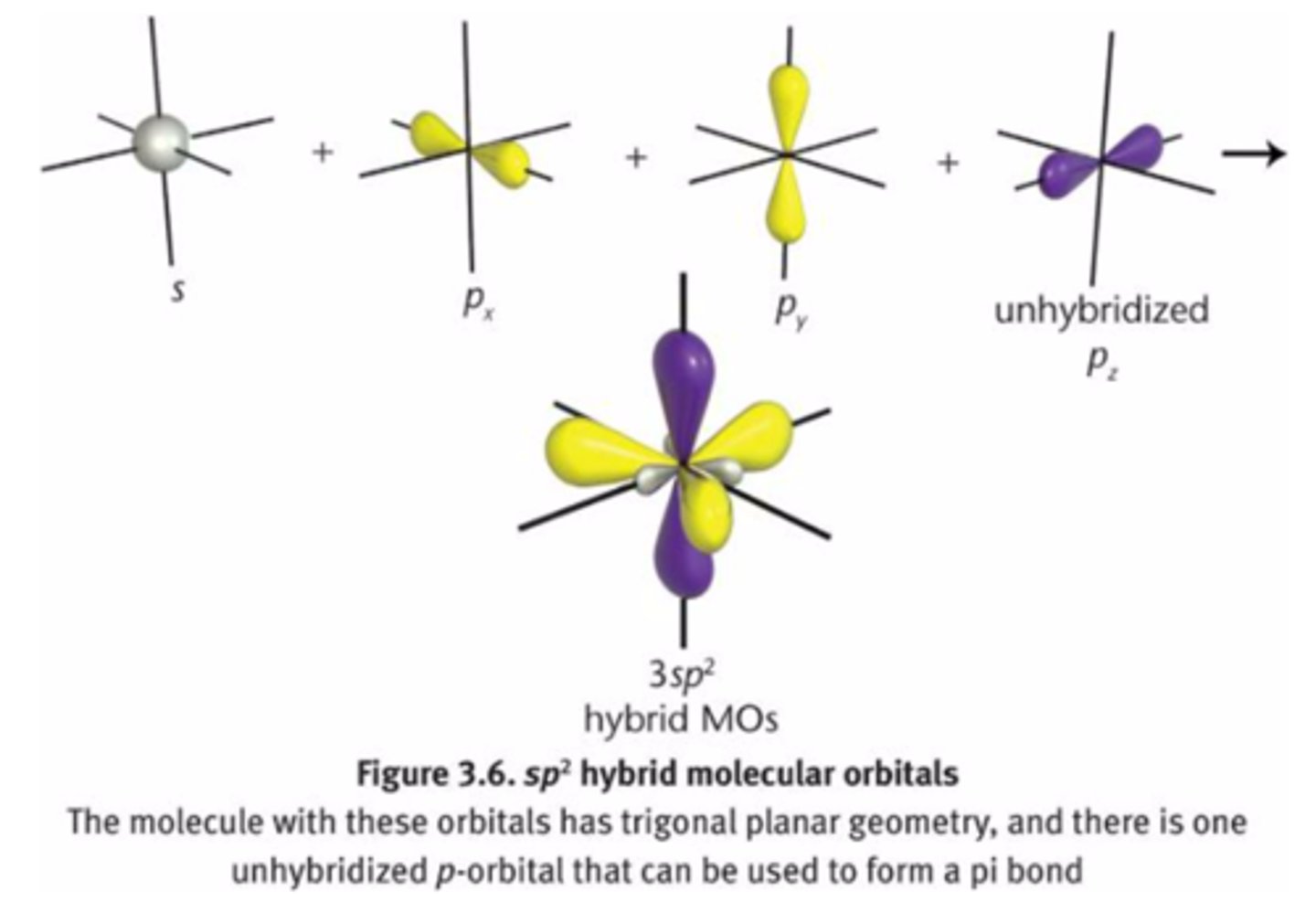
sp2
-double bond
-trigonal planar
33% s character
67% p character
one s-orbital
two p-orbitals
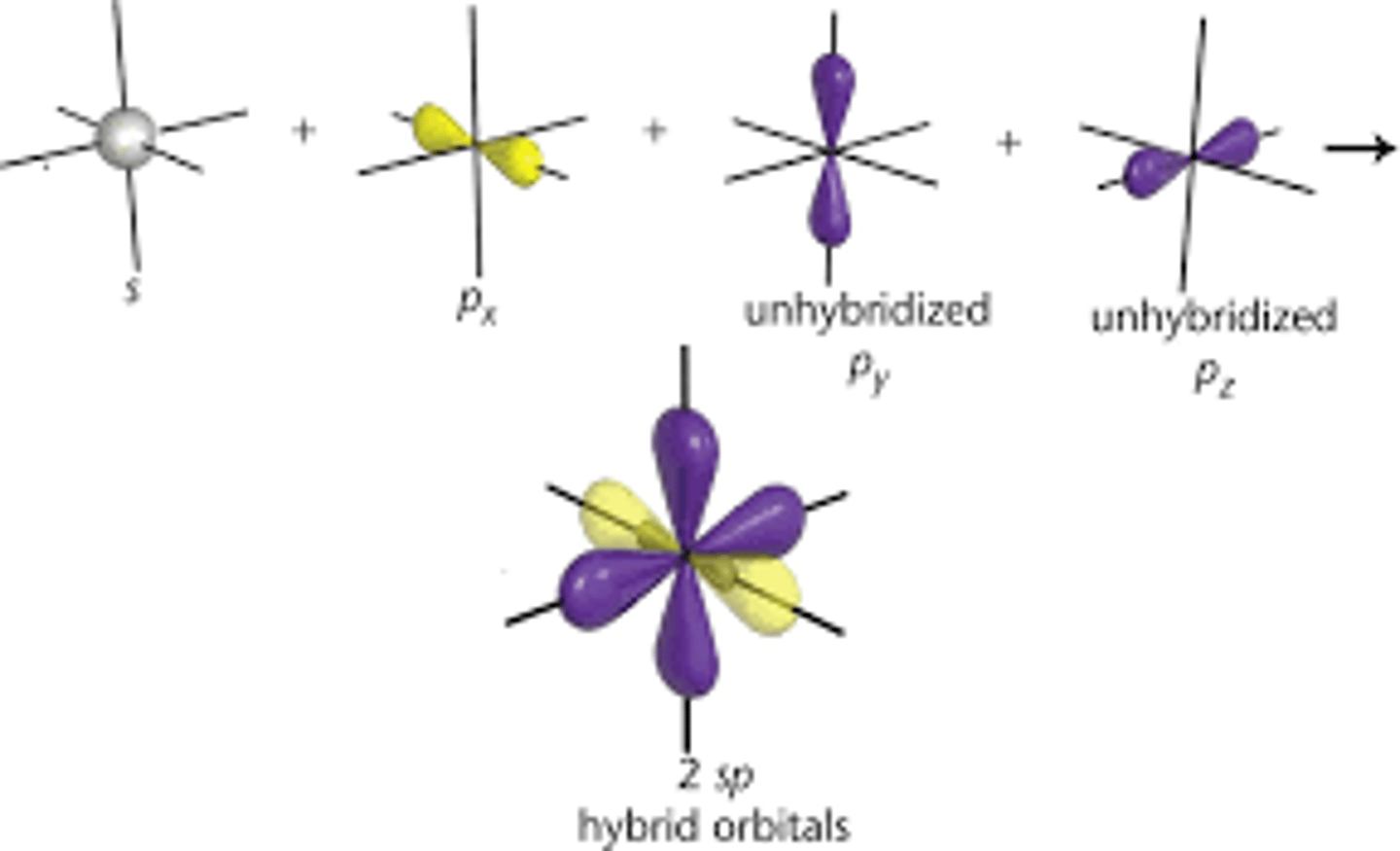
sp
-triple bonds
-linear geometry
one s
one p
50% s character
50% p character
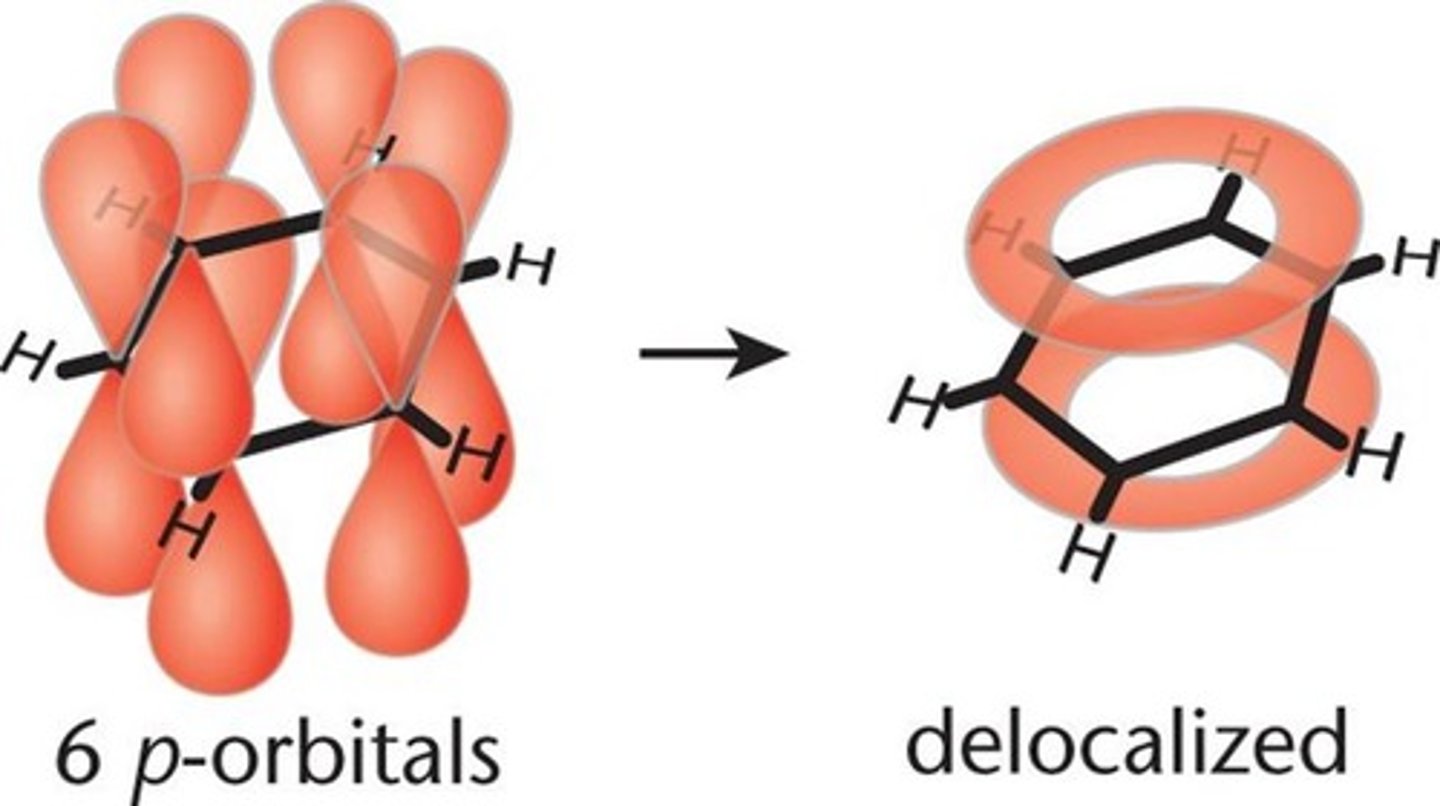
Conjugation
-in resonance
-requires alternating single + multiple bonds because this pattern aligns a number of unhybridized p-orbitals down the backbone of a molecule
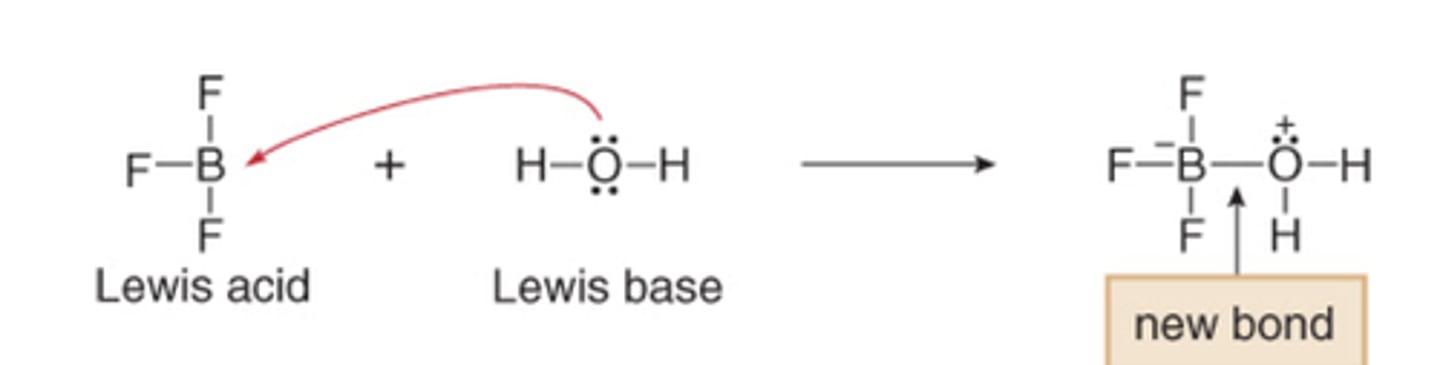
Lewis Acid
-electron pair acceptor
-electrophile
Lewis Base
-electron pair donor
-nucleophile
Bronsted-Lowry acid
proton donor (H+)
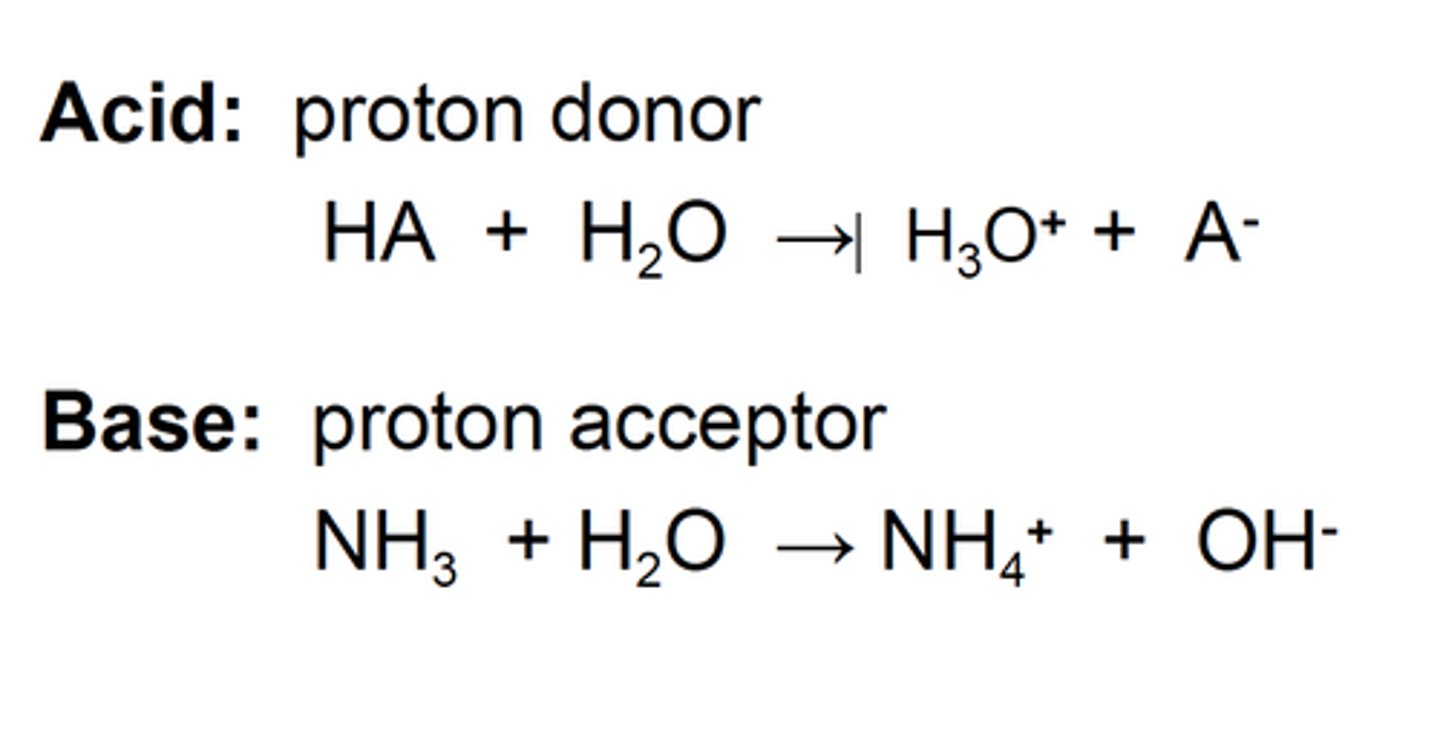
Bronsted-Lowry base
proton acceptor
Rules for acidity
1) bond strength decreases down the periodic table --> acidity increases
2) more electronegative = higher acidity
acid dissociation constant
measures the strength of an acid in solution
pka=-log ka
(more acidic-->more (-)pka--->more smaller)
(more basic--->more (+)pka--->larger)
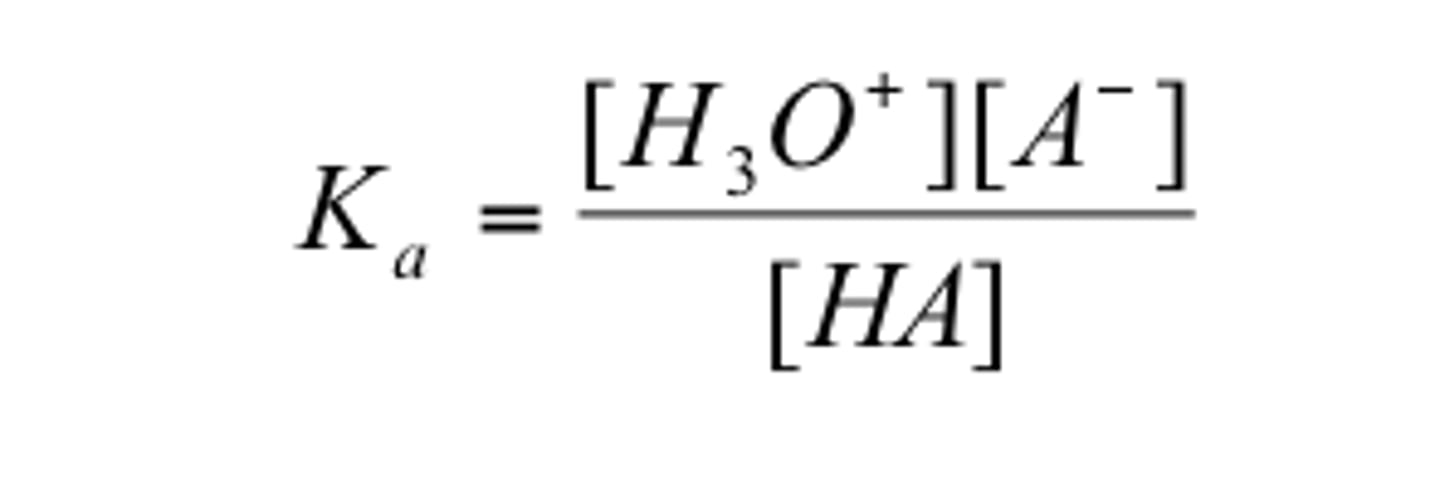
Functional groups that act as acids
- alcohols
- aldehydes
- ketones (at the α-carbon)
- carboxylic acids + most derivatives
- easier to target with basic (nucleophilic) reactants because they readily accept a lone pair
Functional groups that act as bases
- amines
- amides
Factors that determine nucleophilicity
-Charge: increases with more negative charge
- Nu decreases as EN increases
-Solvent: Protic solvents can hinder nucleophilicity by protonating the nucleophiles or through hydrogen bonding
Nucleophile
- nucleus loving
-lone pairs or pi
-good bases (more basic-->more reactive)
-most Nu: C, H, O, N
-more Nu, more (-)
-more bulkier, less Nu
-Strong Nu: RNH2, HO-, RO-, CN-, N3-
-weak Nu: H2O, ROH, RCOOH
-fair Nu: NH3, RCO2-
In polar protic solvents
*nucleophilicity increases down the periodic table
- carbox. acids, ammonia/amines, water/alcohols
- can hydrogen bond
In polar aprotic solvents
*nucleophilicity increases up the periodic table
- DMF, DMSO, acetone
- can't hydrogen bond
Electrophile
-electron loving
-accepts e- pair (+)
-act as lewis acid (acceptor)
-alcohol, aldehydes, ketones, CA and their derivatives
*most reactive electrophilicity to least:
anhydride-->CA--->esters--->amide
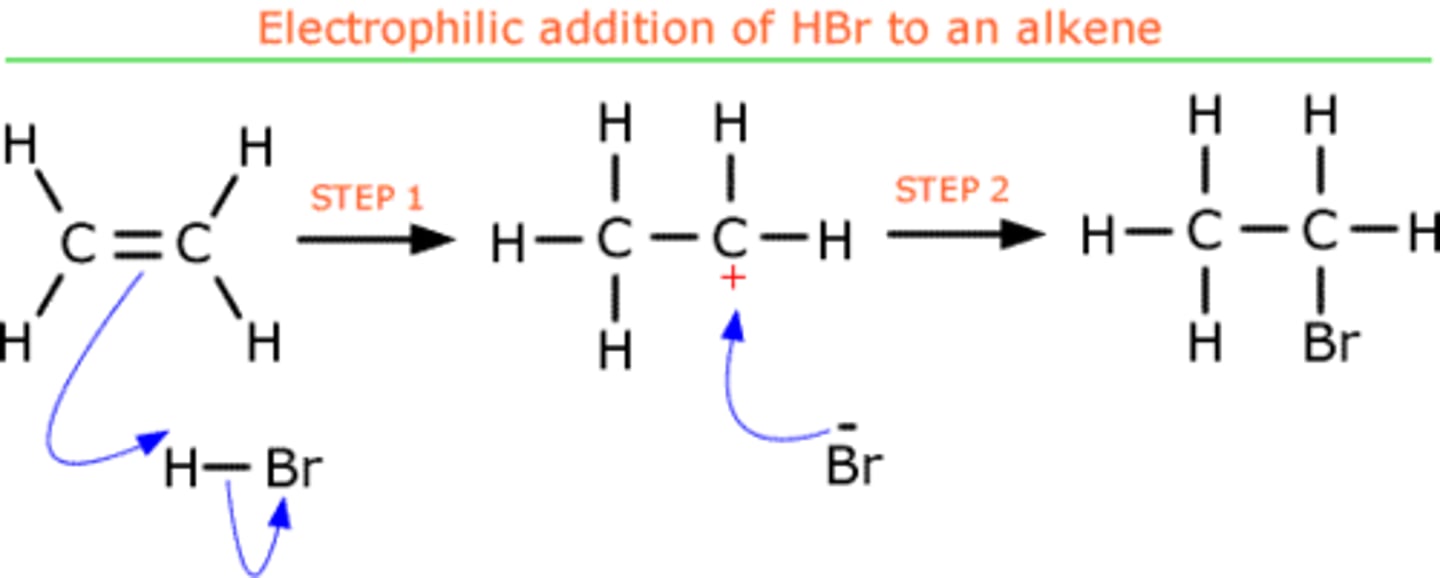
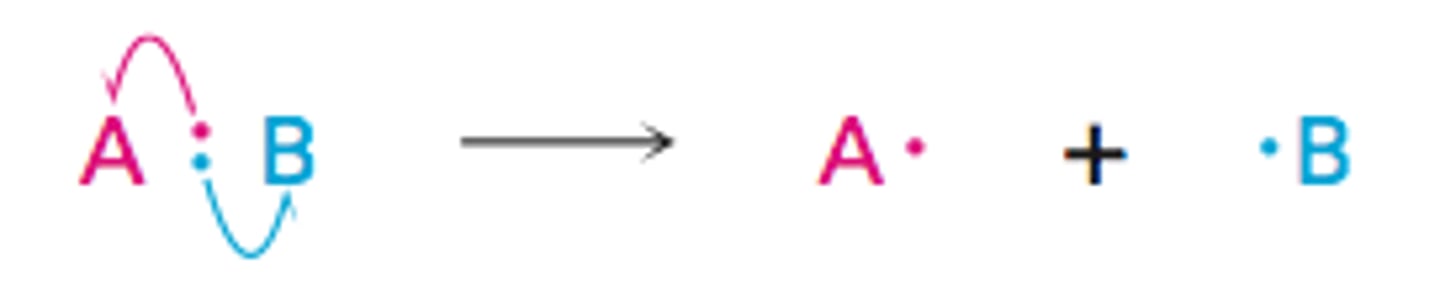
Heterolytic reactions
- bond is broken and both electrons are given to one of the two products
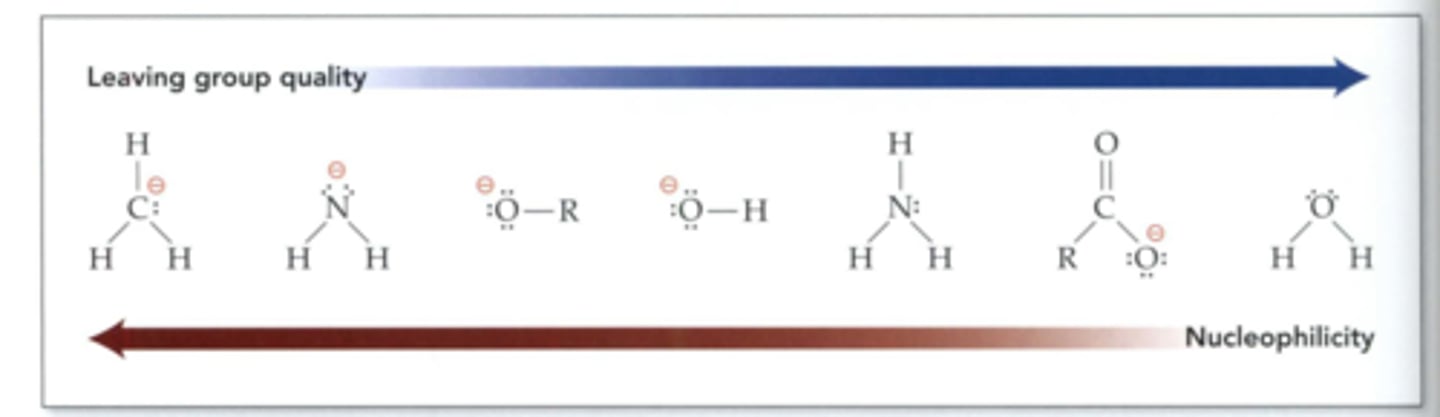
Good leaving groups
-weak bases: I-, Br-, Cl-
-alkanes and H+: NEVER serve as LG
-in substitution reactions: weaker base (LG) is replaced by stronger base (Nu)
SN1 reactions
(unimolecular nucleophilic substitution)
- 2 steps
1) leaving group leaves and generates a carbocation (rate limiting/slower step)
2) nucleophile then attacks the carbocation and the substitution product results
-Kinetics: reaction rate = k[electrophile]
-Favoring Conditions: non-basic, weaker nucleophiles favor unimolecular substitutions.
![<p>- 2 steps</p><p>1) leaving group leaves and generates a carbocation (rate limiting/slower step)</p><p>2) nucleophile then attacks the carbocation and the substitution product results</p><p>-Kinetics: reaction rate = k[electrophile]</p><p>-Favoring Conditions: non-basic, weaker nucleophiles favor unimolecular substitutions.</p>](https://knowt-user-attachments.s3.amazonaws.com/5c7896c7-d708-42ca-b68d-3ff4202d5924.jpg)
SN2 reactions
(bimolecular nucleophilic substitution)
1 step --> concerted reaction
- nucleophile attacks the compound at the same time as the leaving group leaves
Kinetics: reaction rate = k[nucleophile][electrophile]
Solvent: favored by polar aprotic solvents (no H-bonding)
Favoring Conditions: strong non-bulky nucleophile will favor SN2 reactions
*backside attack:
- nuc. must be strong
- substrate cannot be sterically hindered
- less substituted = more reactive
![<p>1 step --> concerted reaction</p><p>- nucleophile attacks the compound at the same time as the leaving group leaves</p><p>Kinetics: reaction rate = k[nucleophile][electrophile]</p><p>Solvent: favored by polar aprotic solvents (no H-bonding)</p><p>Favoring Conditions: strong non-bulky nucleophile will favor SN2 reactions</p><p>*backside attack:</p><p>- nuc. must be strong</p><p>- substrate cannot be sterically hindered</p><p>- less substituted = more reactive</p>](https://knowt-user-attachments.s3.amazonaws.com/60e5a4b2-86dc-4898-becc-beee4cfdd9c7.jpg)
Oxidation
- loss of electrons
- increasing the number of bonds to oxygen or other heteroatoms (besides carbon + hydrogen)
Reduction
- gain in electrons
- increasing number of bonds to hydrogen
oxidizing agent
- element or compound in an oxidation-reduction reaction that accepts an electron from another species
- gaining electrons--->reduced
- good oxidizing agents have a high affinity for electrons (O2, O3, Cl2) or
- high oxidation states (Mn 7+, MnO4-, Cr 6+ in chromate CrO4 2-)
Reducing agents
- reducing agents often contain metals bonded to a large number of hydrides
-Na+, Mg, Al, Zn (low EN and low IE)
-good reducing agents: LiAlH4, NaH, CaH2, NaBH4 (b/c contain H-)
Chemoselectivity
-preferential reaction of one functional group in the presence of other functional groups
Highest priority functional group
- is most likely to be acted on by redox reagents and during nucleophile and electrophile reactions
- Ex: COOH is most oxidized, has the most Electronegative groups around it and therefore has a larger partial positive charge on it
Order: COOH, aldehyde or ketone, alcohol or amine
Steric hindrance
- prevention of reactions at a particular location within a molecule due to the size of substituent groups
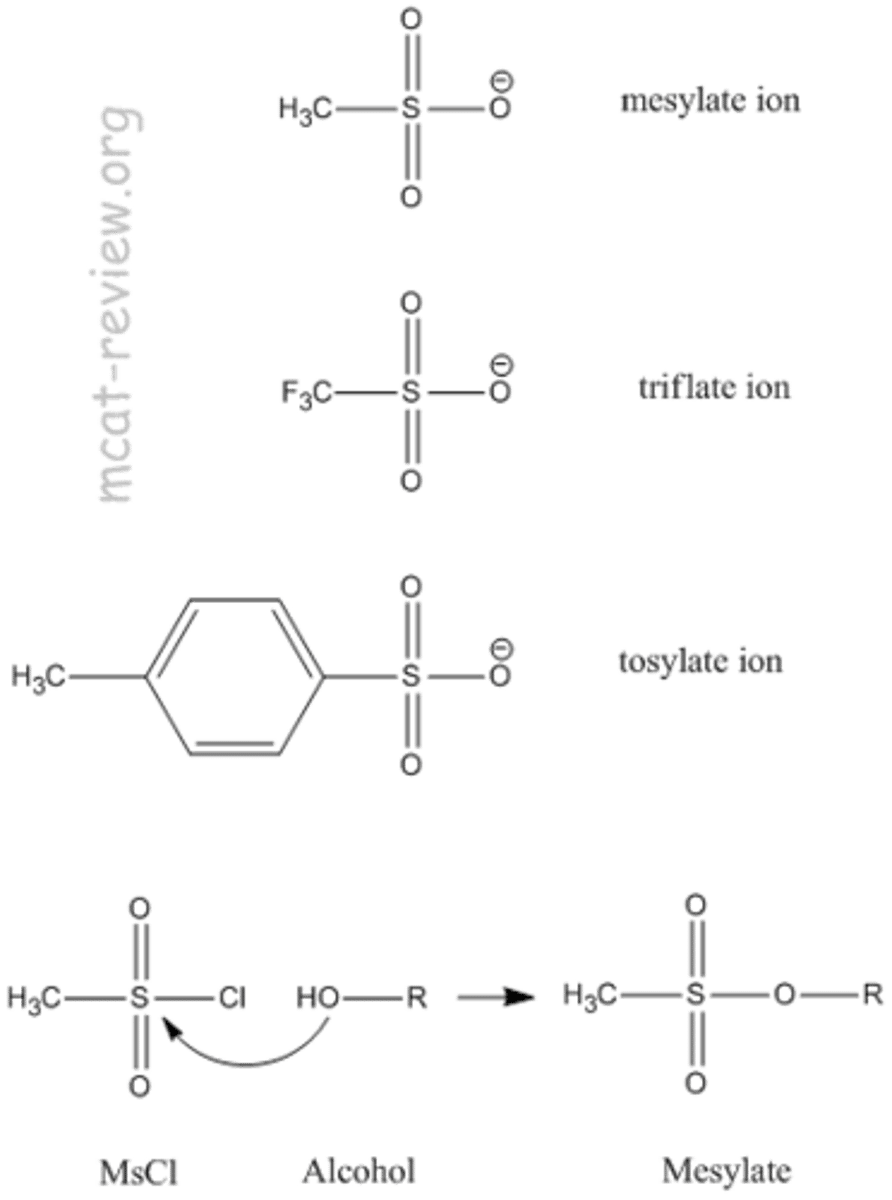
Protecting Groups for alcohols
mesylate and tosylates
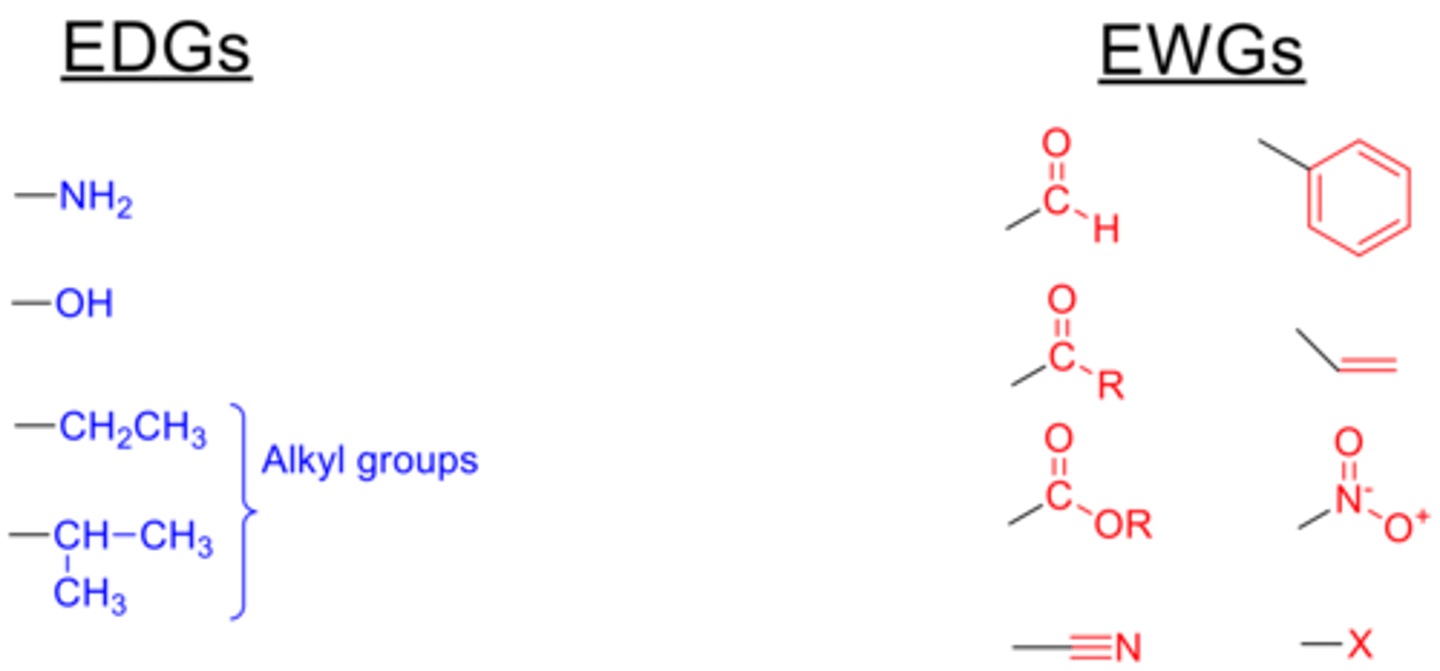
electron withdrawing groups
increase acidity
-double bond oxygen more EW than single bond
electron donating groups
decrease acidity
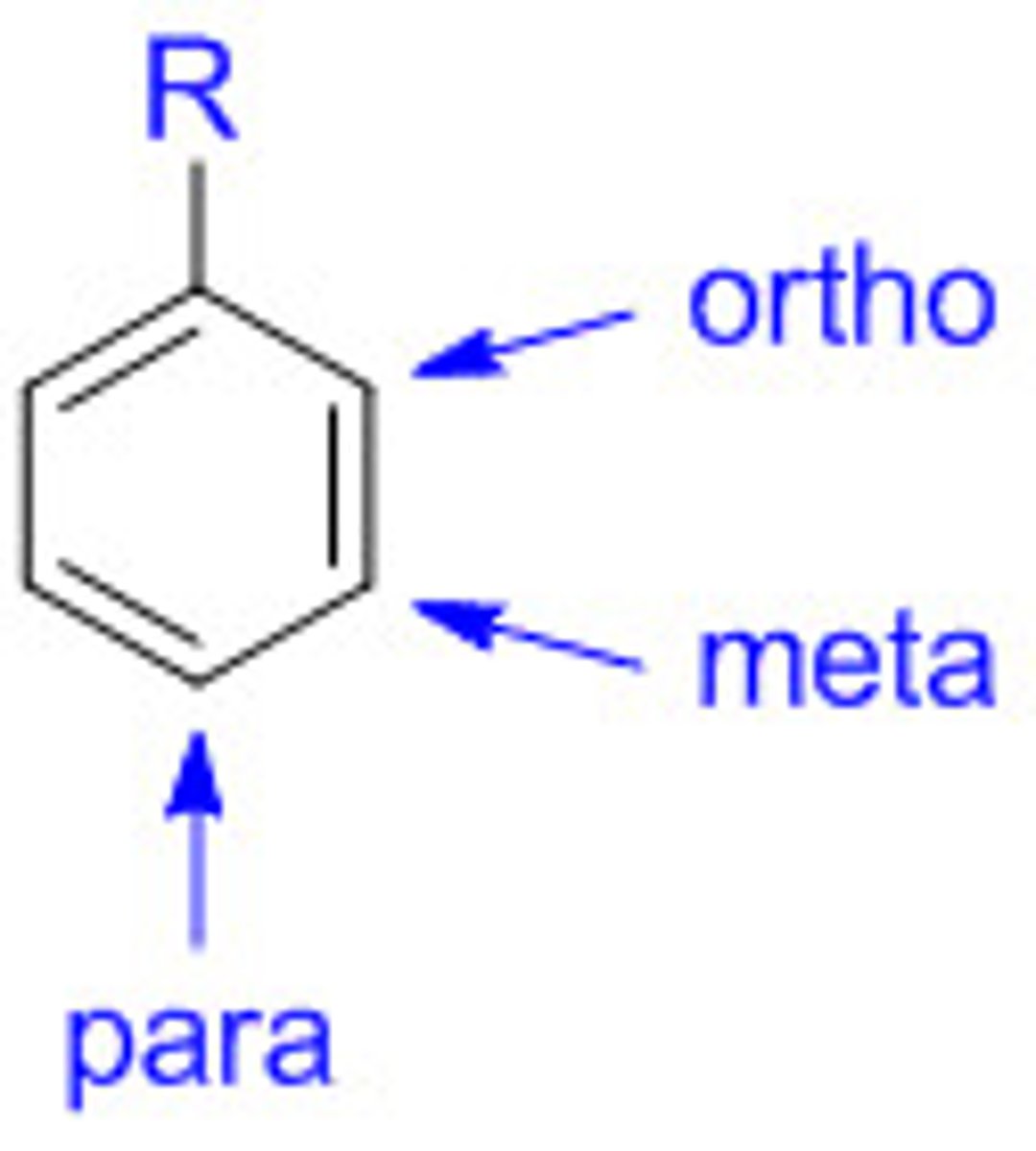
ortho, meta, para
hydrogen bonding
increase mp
increase bp
increase solubility in H2O
PCC
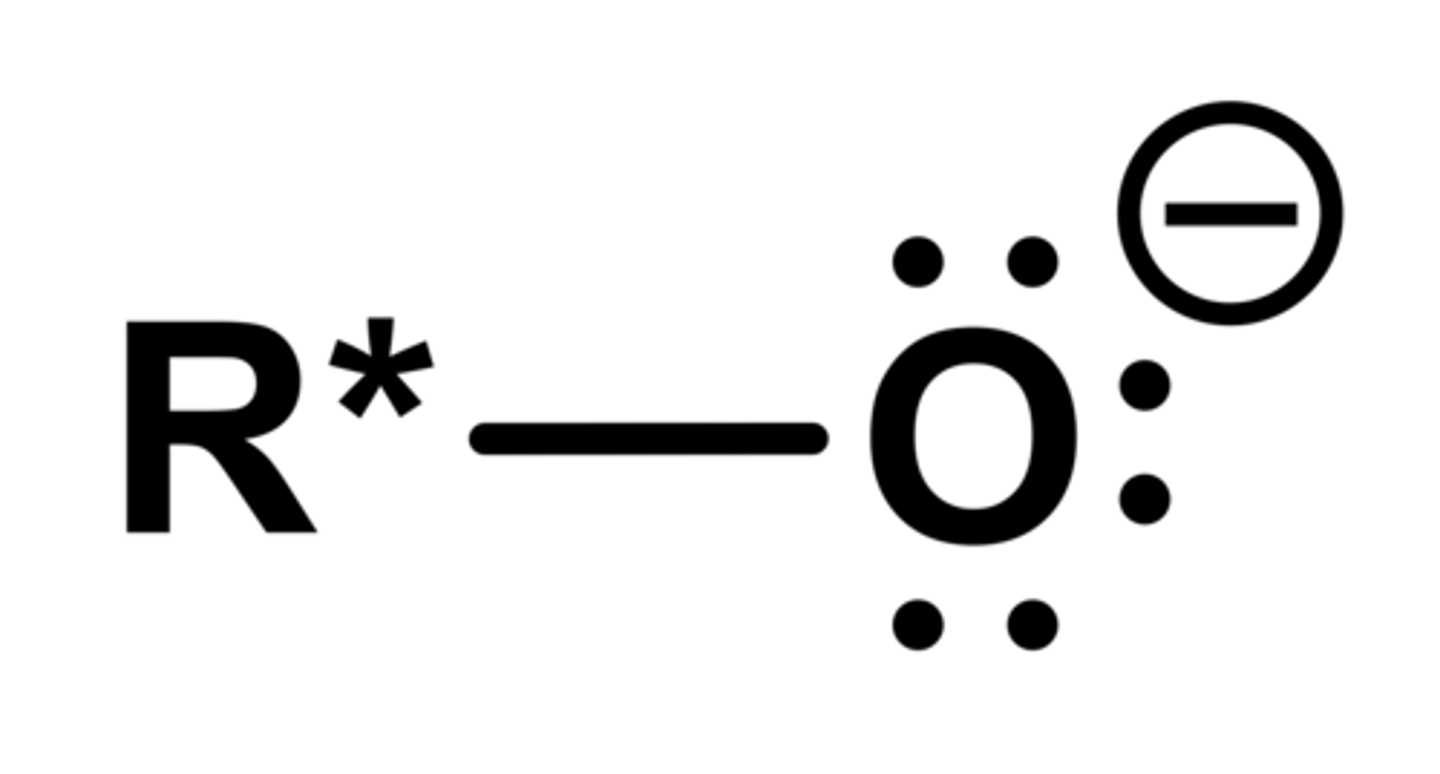
alkoxide anion
charges like to spread out as much as possible. Acidity decreases as more alkyl groups are attached b/c they are electron donating, which destabilizes the alkoxide anion
Resonance or electron withdrawing groups stabilize the alkoxide anion, making the alcohol more acidic
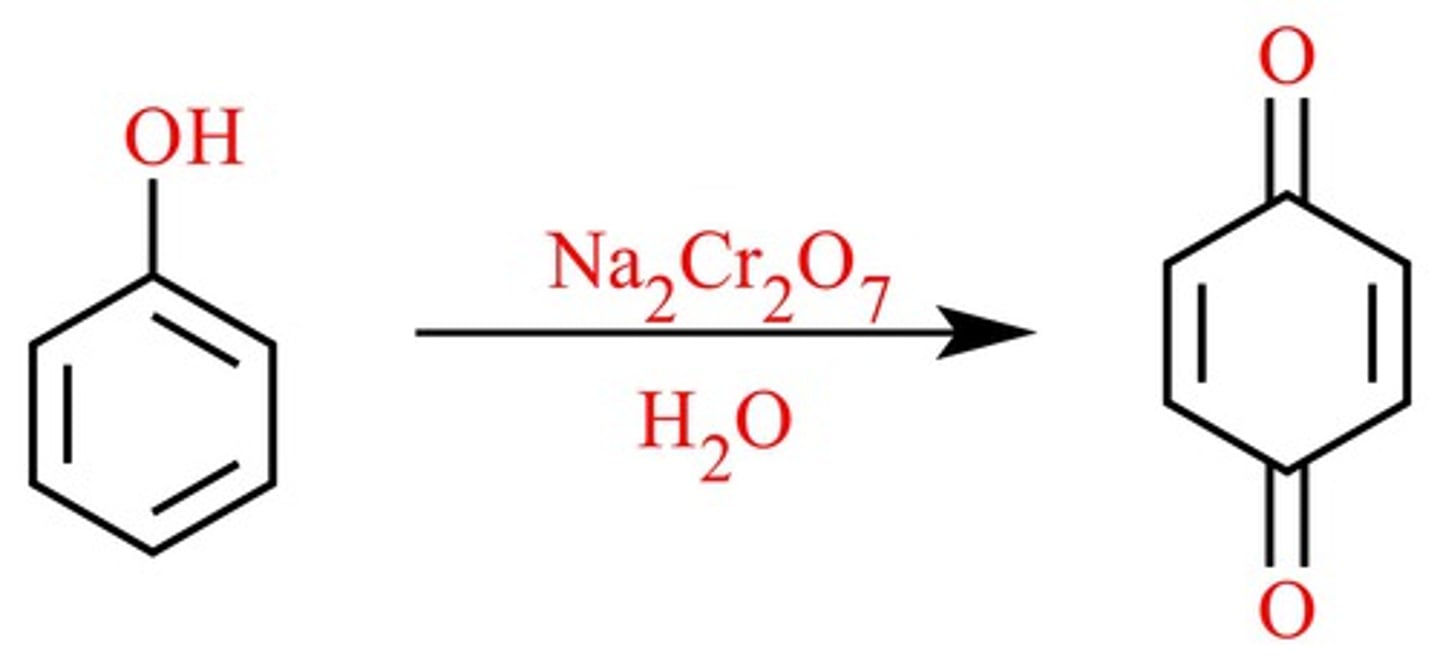
Quinones
-electron acceptor in ETC
Hydroxyquinones
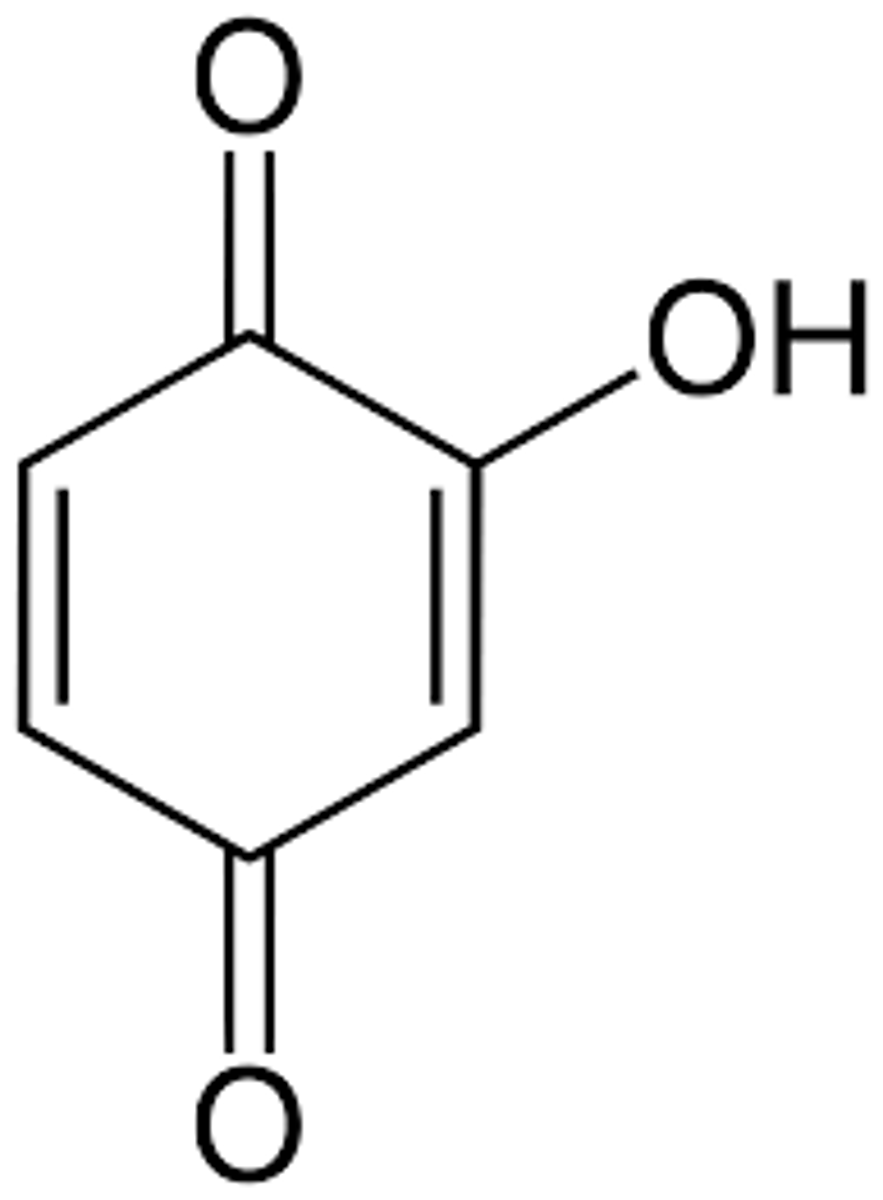
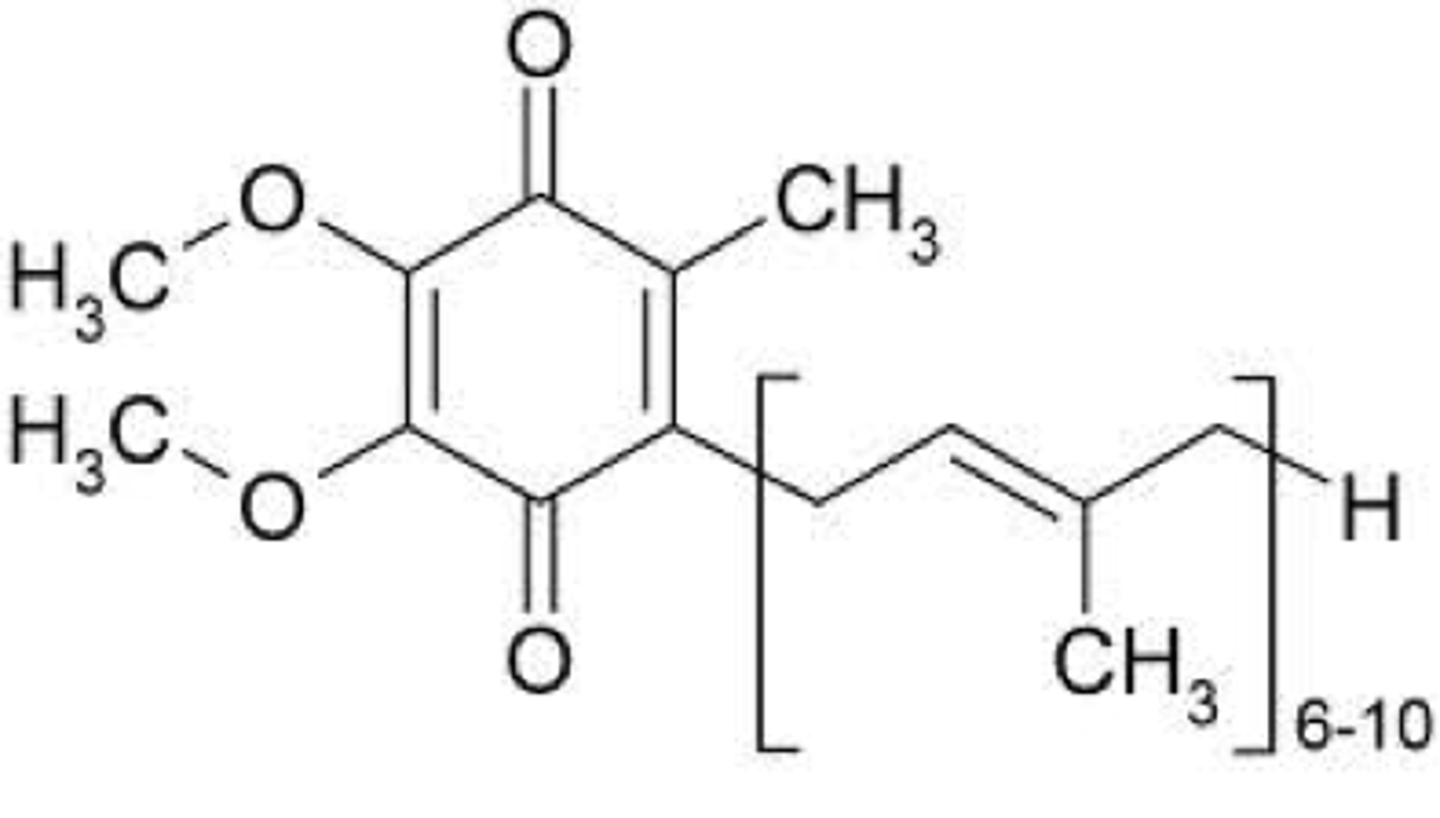
Ubiquinone (coenzyme Q)
-lipid soluble (b/c of long alkyl chain)
-electron carrier in ETC.
-Ubiquinol: reduced form (with -OH instead of C=O) when it picks e-
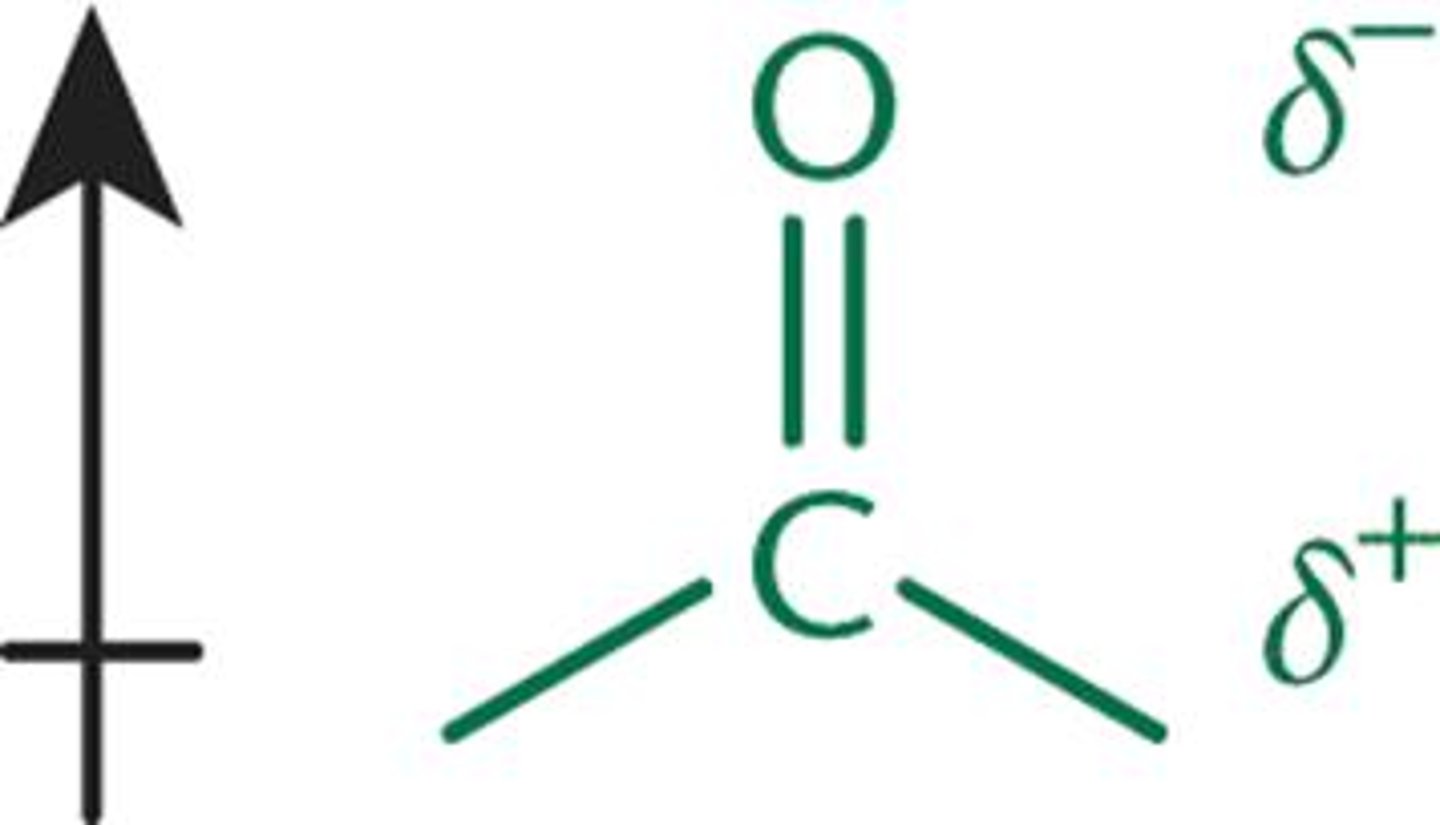
Ketone/Aldehyde physical property
- dipole of carbonyl is stronger of alcohol bc of C=O
- BP are elevated because of increased intermolecular attractions, less than OH's however bc no H-bonding
- act as electrophiles, good targets for nucleophiles: due to e-withdrawing properties of the carbonyl oxygen, leaves partial positive charge on Carbon
- aldehydes are usually more reactive toward nucleophiles bc less steric hindrance + fewer e-donating alkyl groups
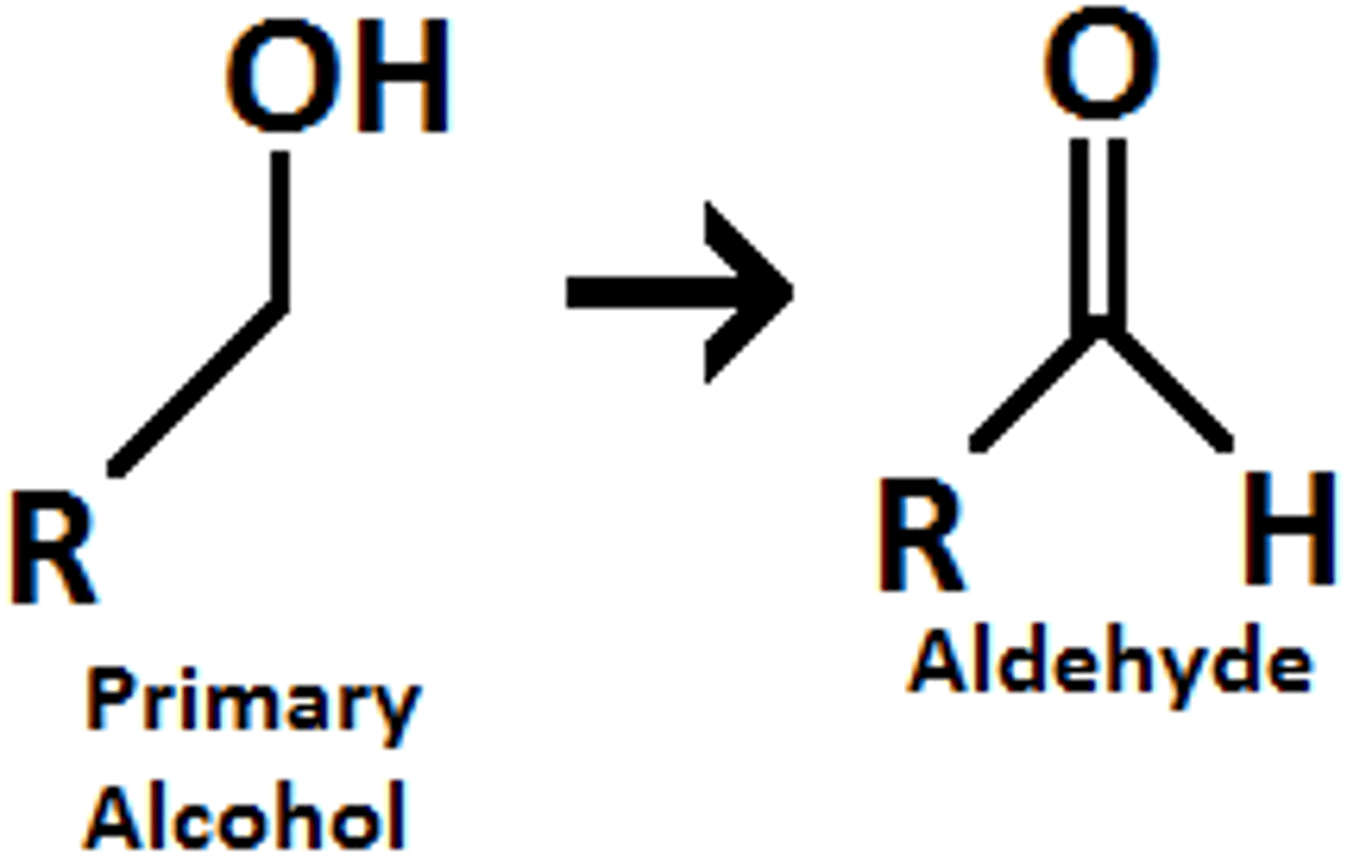
Formation of aldehyde
- obtained from partial oxidation of a primary alcohol, only by PCC!
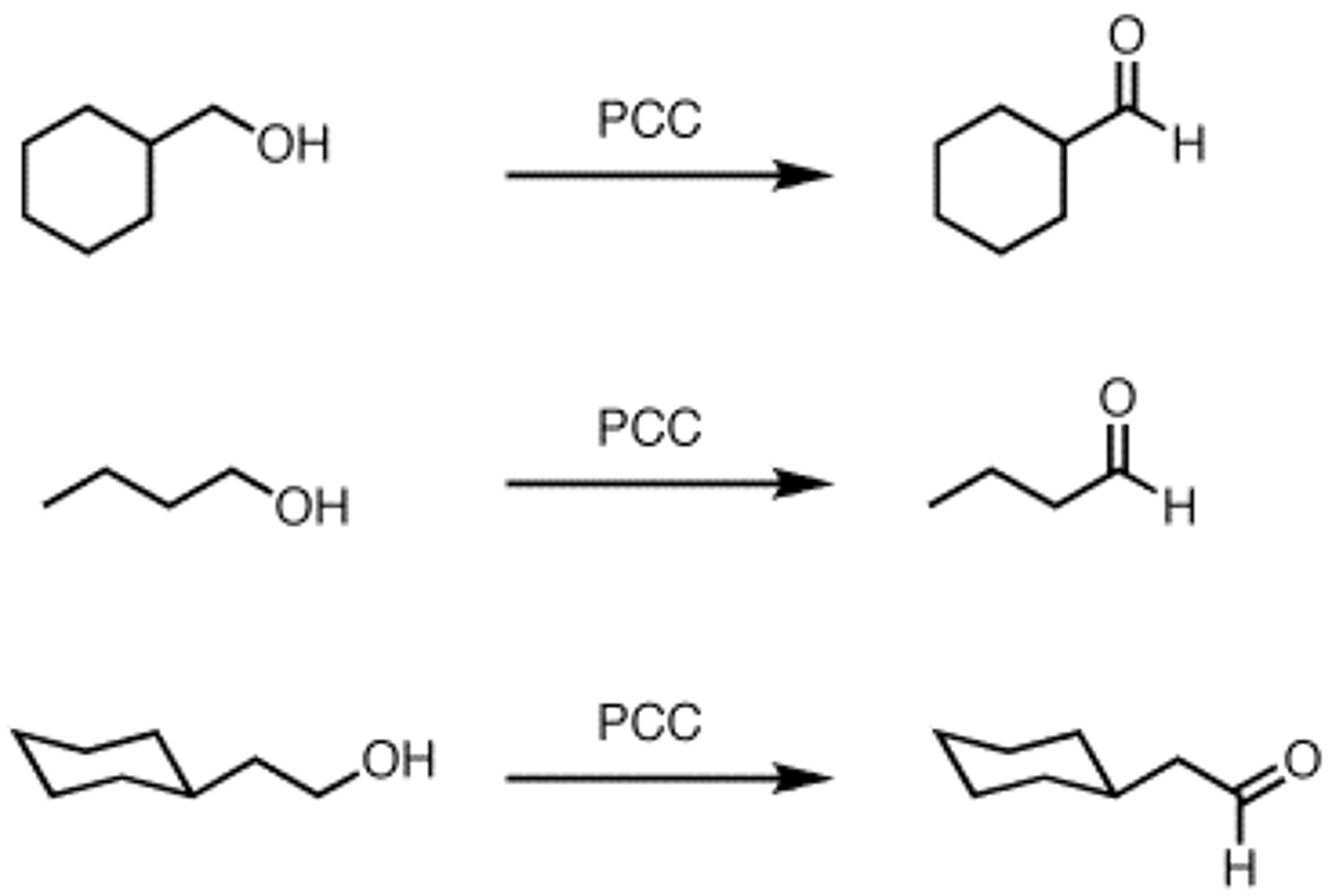
Formation of a ketone
- oxidation of a secondary alcohol
- Can use:
--> Na2Cr2O7
--> K2Cr2O7
--> CrO3
--> PCC
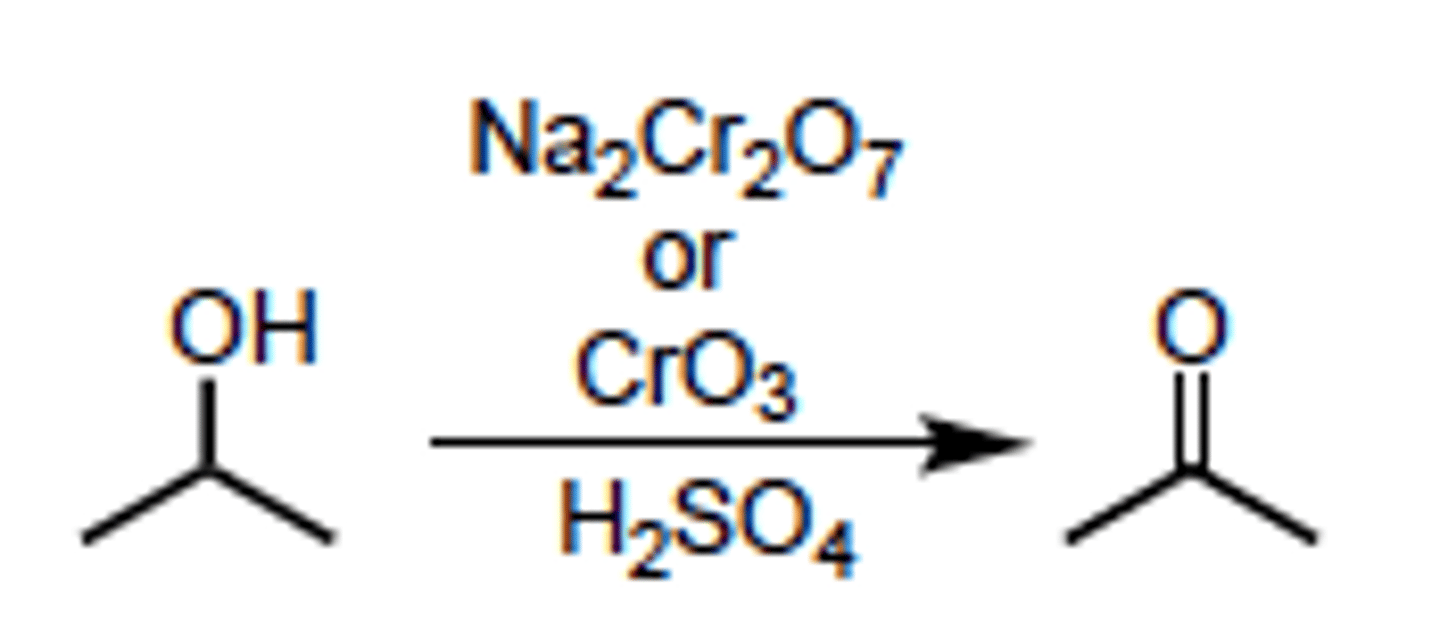
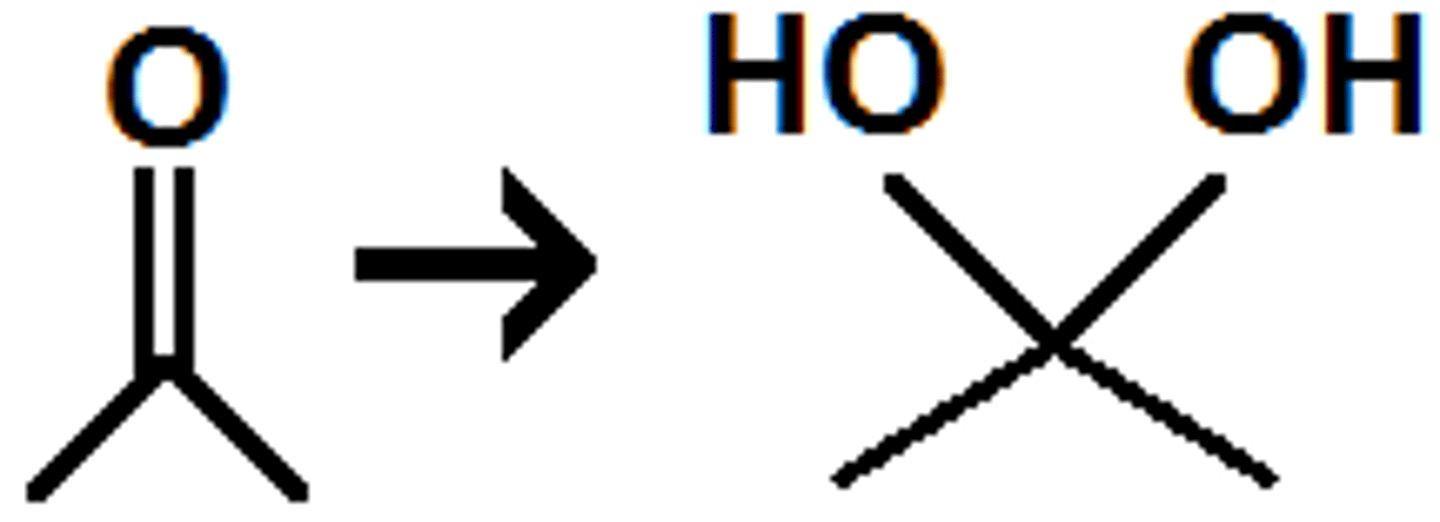
Hydration
- in presence of water, aldehydes + ketones react to form geminal diols
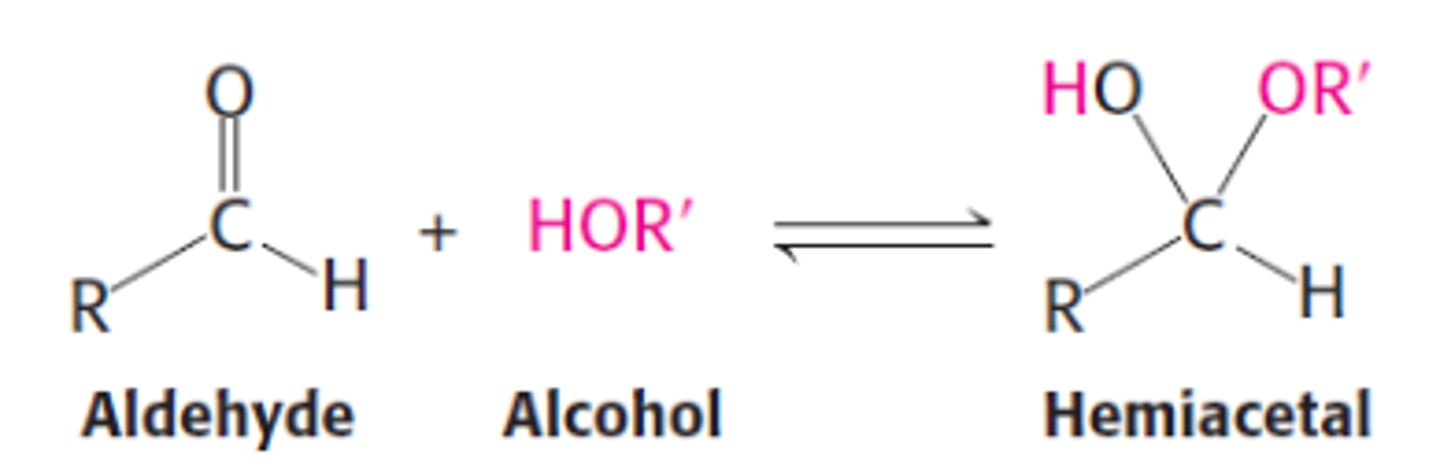
When an aldehyde or ketone is treated with 1 equivalent of an alcohol(Nu)
- product is a hemiacetal or hemiketal
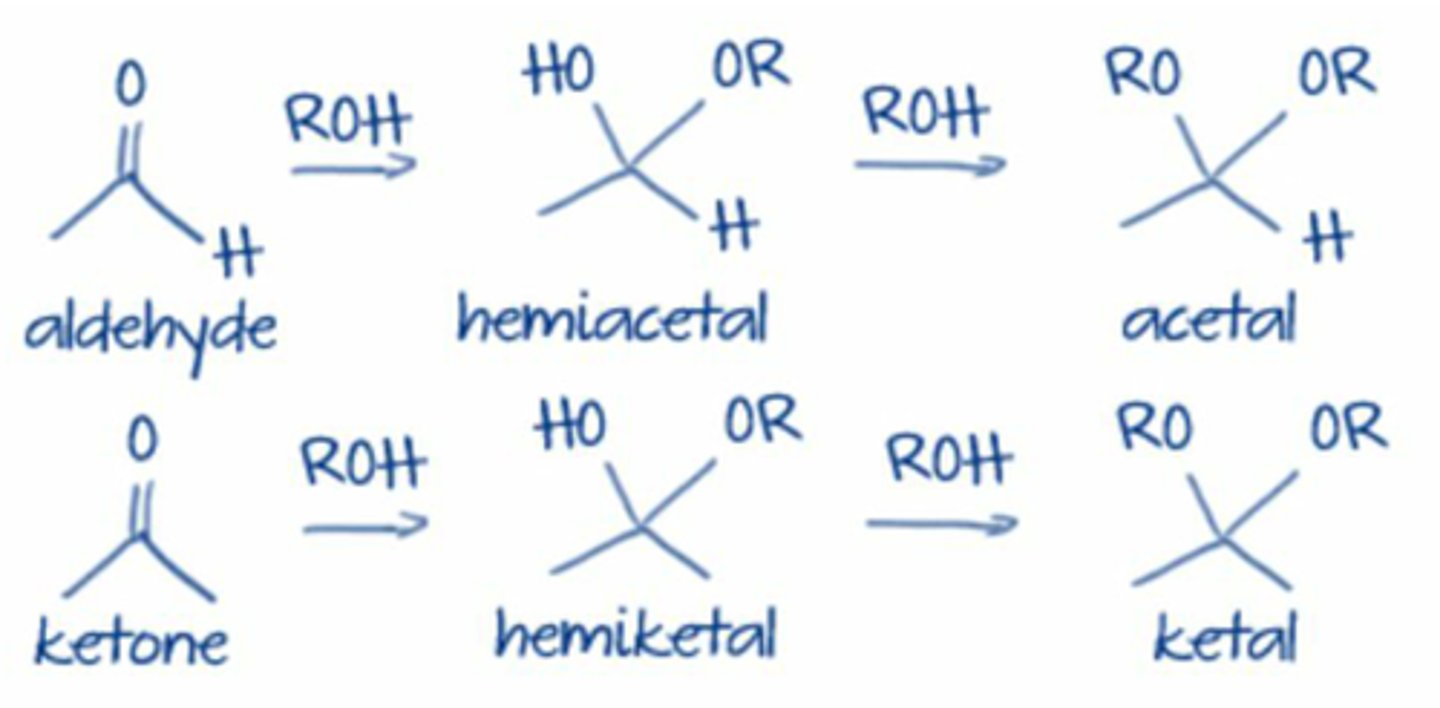
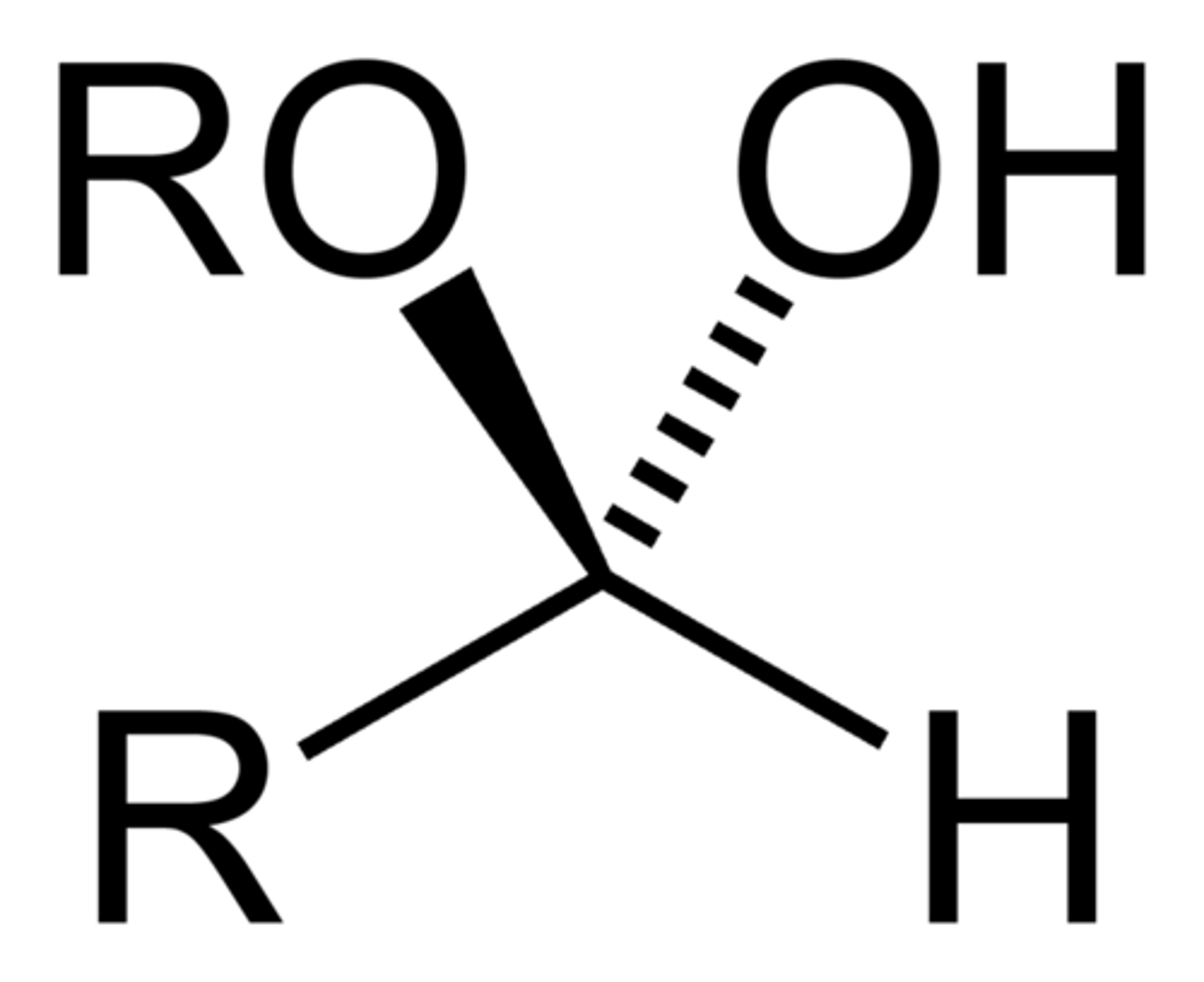
hemiacetal
hemiketal
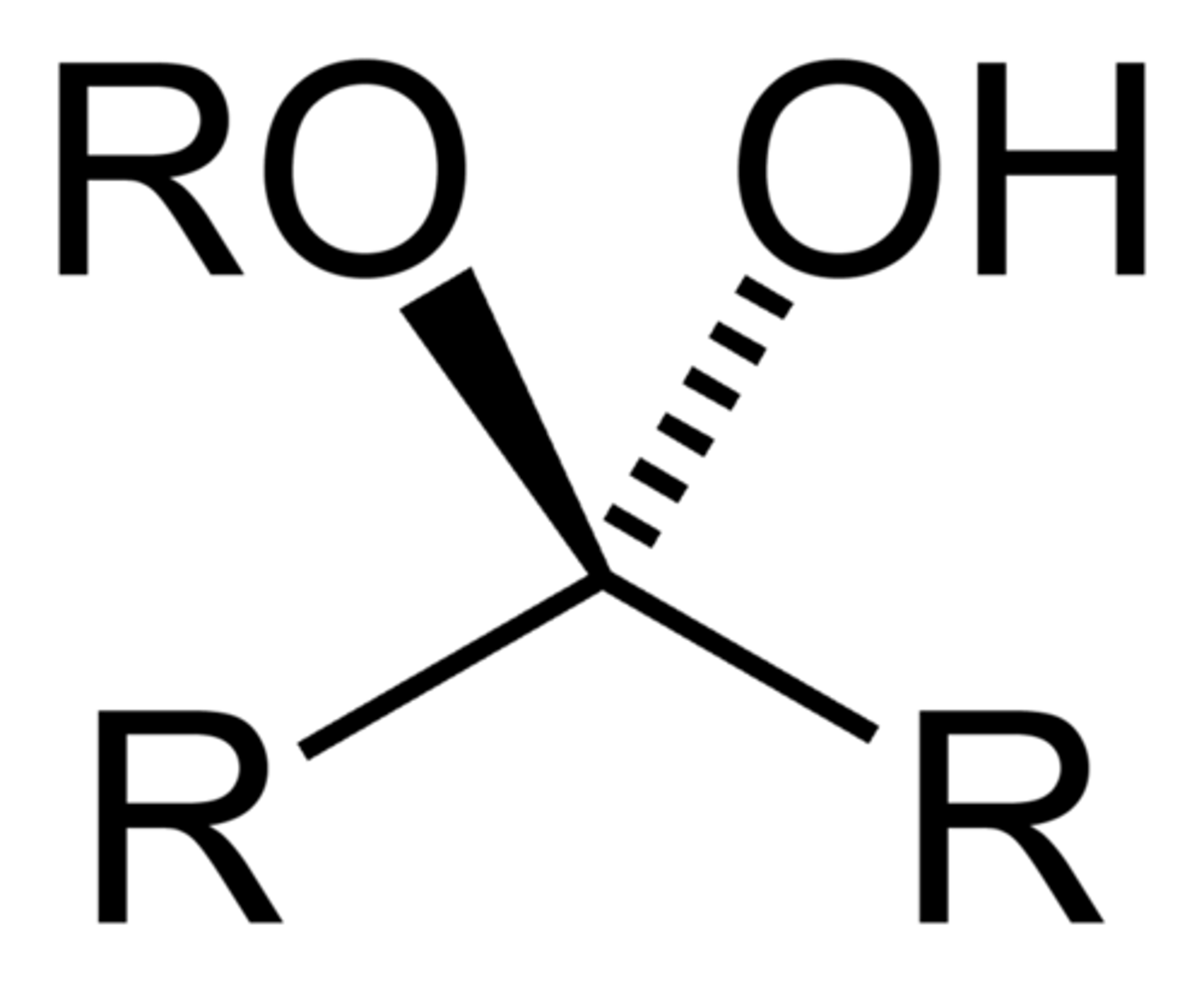
Hemiacetal Formation
- recognize by retention of -OH group