8.1 rate of reaction
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
Rate =
Change in amount of reactants or products / time
Define activation energy
The minimum energy that colliding particles must possess for a collision to be effective
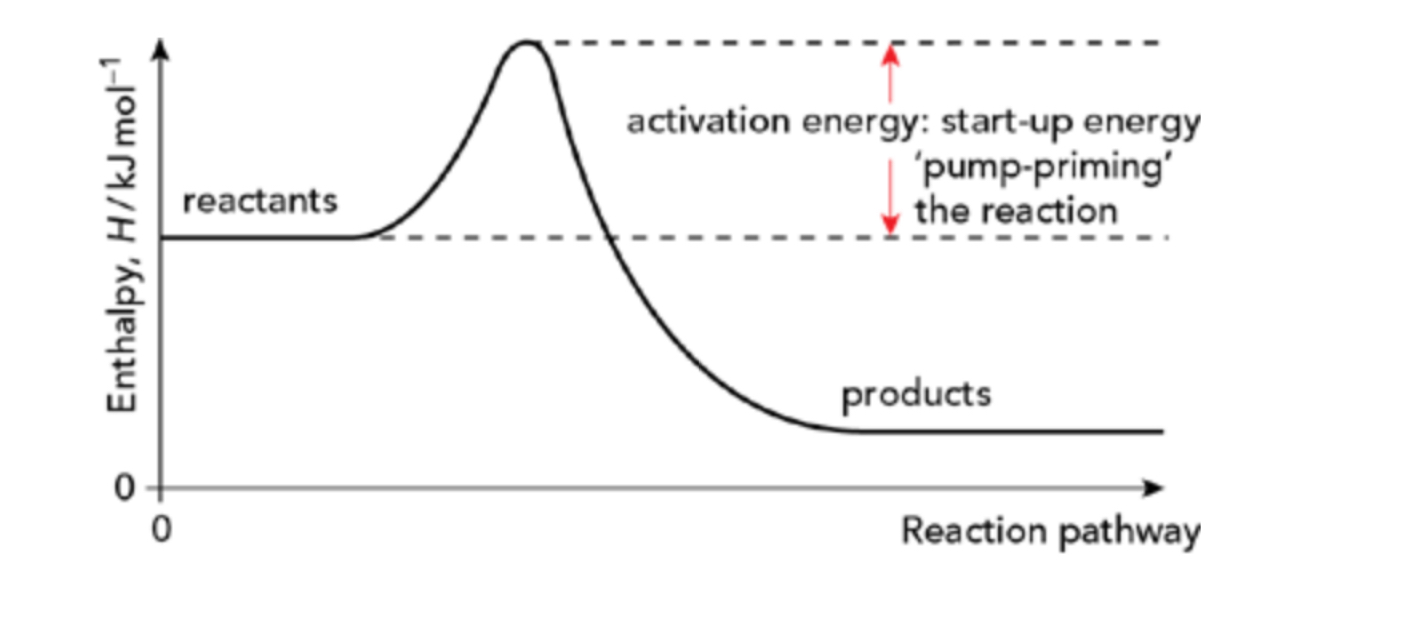
Draw the reaction pathway for an exothermic reaction
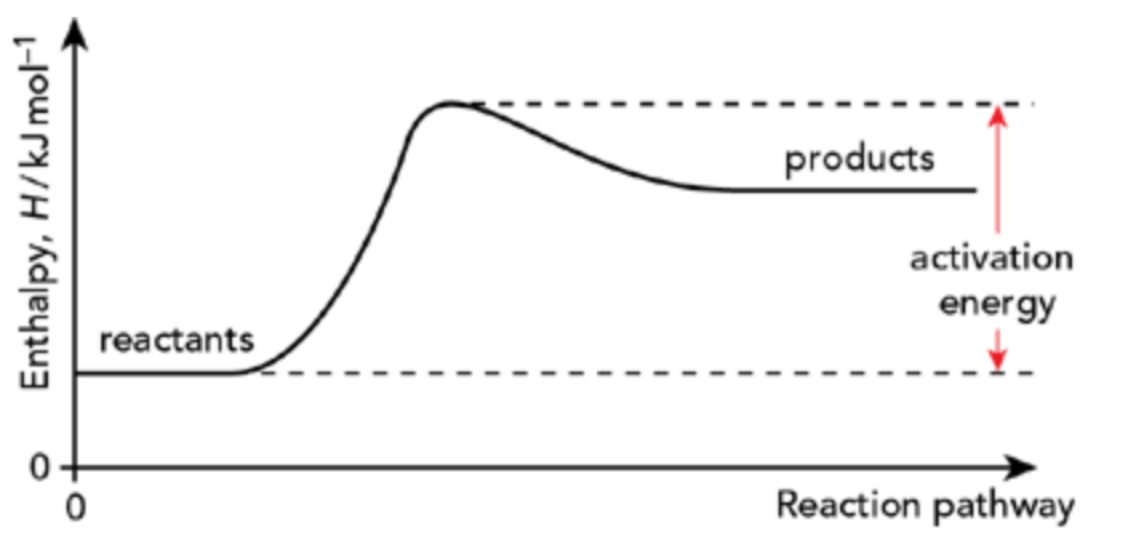
Draw the reaction pathway for an endothermic reaction
According to collision theory, a reaction will speed up if:
the frequency of collisions increases
The proportion of particles with energy greater than the activation energy increases
Define catalyst
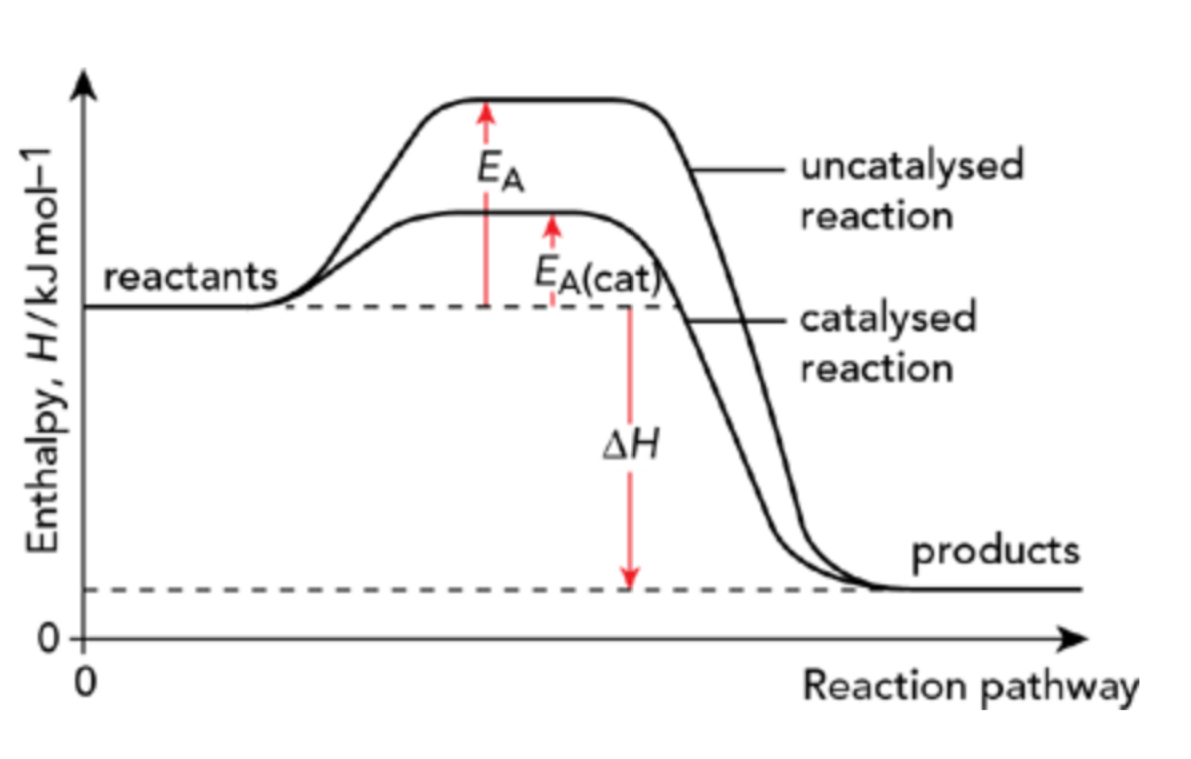
A substance that speeds up the rate of reaction by providing an alternative reaction pathway and thus lowering the activation energy
How does the use of a catalyst affect the reaction pathway?
What does the collision theory state?
For a chemical reaction to take place, particles need to collide with each other in correct orientation with enough energy
How does increasing concentration affect the rate of reaction?
causes an increased frequency of collisions
Therefore an increased frequency of successful collisions
Thus an increased rate of reaction
How does increasing pressure affect the rate of reaction?
the molecules have less space in which they can move
This means that the number of successful collisions increases due to an increased collision frequency
An increase in pressure therefore increases the rate of reaction